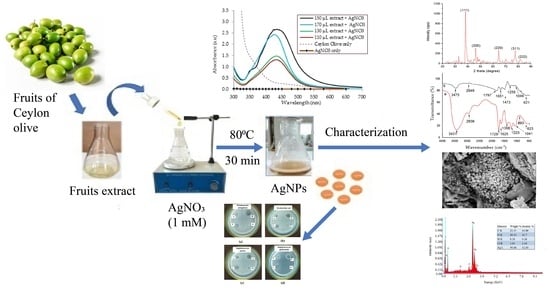
Synthesis of Silver Nanoparticles Using Green Reducing Agent: Ceylon Olive (Elaeocarpus serratus): Characterization and Investigating Their Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ceylon Olive Fruit Extract
2.3. Synthesis of AgNPs with Optimization of Process Parameters
2.3.1. Effect of the Volume of Ceylon Olive Fruit Extract on the Formation of Silver Nanoparticles
2.3.2. Effect of Temperature on the Formation of AgNPs
2.3.3. Effect of pH on the Formation of AgNPs
2.4. Characterization of AgNPs
2.4.1. Particle Size Analysis
2.4.2. X-ray Diffraction (XRD) Structural Analysis
2.4.3. Scanning Electron Microscopy Equipped with Energy Dispersive Spectroscopic (SEM-EDX) Analysis
2.4.4. Fourier Transform Infrared (FTIR) Analysis
2.5. Antimicrobial Activity
Minimum Inhibitory Concentration (MIC)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of AgNPs
3.1.1. SEM-EDX Analysis
3.1.2. XRD Analysis
3.1.3. FTIR Analysis
3.1.4. Particle Size Analysis
3.2. Visual Observation of the Formation of AgNPs
3.3. Investigatation of the Formation of AgNPs with a UV Spectrophotometer
3.3.1. Effect of Volume of Extract on the Formation of AgNPs
3.3.2. Effect of Temperature
3.3.3. Effect of pH of Medium for the Formation of the AgNPs
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linklater, D.P.; Baulin, V.A.; Le Guével, X.; Fleury, J.B.; Hanssen, E.; Nguyen, T.H.P.; Juodkazis, S.; Bryant, G.; Crawford, R.J.; Stoodley, P.; et al. Antibacterial Action of Nanoparticles by Lethal Stretching of Bacterial Cell Membranes. Adv. Mater. 2020, 32, 2005679. [Google Scholar] [CrossRef] [PubMed]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Abdullah, C.A.C.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K. Prospects of Nanostructure Materials and Their Composites as Antimicrobial Agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 1–30. [Google Scholar] [CrossRef] [PubMed]
- El-kader, F.H.A.; Hakeem, N.A.; Elashmawi, I.S.; Menazea, A.A. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Synthesis and Characterization of PVK / AgNPs Nanocomposites Prepared by Laser Ablation. Spectrochim. ACTA PART A Mol. Biomol. Spectrosc. 2016, 138, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.P.; Yadav, R.M.; Singh, D.P. Mechanical Milling: A Top Down Approach for the Synthesis of Nanomaterials and Nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Benetti, G.; Cavaliere, E.; Banfi, F.; Gavioli, L. Antimicrobial Nanostructured Coatings: A Gas Phase Deposition and Magnetron Sputtering Perspective. Materials 2020, 13, 784. [Google Scholar] [CrossRef]
- Torrisi, G.; Cavaliere, E.; Banfi, F.; Benetti, G.; Raciti, R.; Gavioli, L.; Terrasi, A. Ag Cluster Beam Deposition for TCO/Ag/TCO Multilayer. Sol. Energy Mater. Sol. Cells 2019, 199, 114–121. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Vigliotta, G.; Picariello, E.; Banfi, F.; Cavaliere, E.; Ciambriello, L.; Gavioli, L. Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications. Coatings 2020, 10, 1238. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B. Potentials in Synthesizing Nanostructured Silver Particles. Microsyst. Technol. 2017, 23, 4345–4357. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.B.; Salimi, M.N.; Letchumanan, I.; Subramaniam, S. Green Synthesized Strontium Oxide Nanoparticles by Elodea Canadensis Extract and Their Antibacterial Activity. J. Nanostructure Chem. 2022, 12, 365–373. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Hamedi, S.; Shojaosadati, S.A. Rapid and Green Synthesis of Silver Nanoparticles Using Diospyros Lotus Extract: Evaluation of Their Biological and Catalytic Activities. Polyhedron 2019, 171, 172. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Miu, B.A.; Dinischiotu, A. New Green Approaches in Nanoparticles Synthesis: An Overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef] [PubMed]
- Abeska, Y.Y.; Cavas, L. Artificial Neural NetworkModelling of Green Synthesis of Silver Nanoparticles by Honey. Neural Netw. World 2022, 32, 1–14. [Google Scholar] [CrossRef]
- Devi, M.; Devi, S.; Sharma, V.; Rana, N.; Bhatia, R.K.; Bhatt, A.K. Green Synthesis of Silver Nanoparticles Using Methanolic Fruit Extract of Aegle Marmelos and Their Antimicrobial Potential against Human Bacterial Pathogens. J. Tradit. Complement. Med. 2020, 10, 158–165. [Google Scholar] [CrossRef]
- Saidu, F.K.; Mathew, A.; Parveen, A.; Valiyathra, V.; Thomas, G.V. Novel Green Synthesis of Silver Nanoparticles Using Clammy Cherry (Cordia Obliqua Willd) Fruit Extract and Investigation on Its Catalytic and Antimicrobial Properties. SN Appl. Sci. 2019, 1, 1368. [Google Scholar] [CrossRef]
- Dutta, T.; Chowdhury, S.K.; Ghosh, N.N.; Chattopadhyay, A.P.; Das, M.; Mandal, V. Green Synthesis of Antimicrobial Silver Nanoparticles Using Fruit Extract of Glycosmis Pentaphylla and Its Theoretical Explanations. J. Mol. Struct. 2022, 1247, 131361. [Google Scholar] [CrossRef]
- Masum, M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus Emblicafruit Extract and Its Inhibitory Action against the Pathogen Acidovorax Oryzaestrain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D.; Kari, Z.A.; Edinur, H.A. Green Synthesis of Silver Nanoparticles Using Diospyros Malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-Np). Nanomaterials 2021, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Rajakannu, S.; Shankar, S.; Perumal, S.; Subramanian, S.; Dhakshinamoorthy, G.P. Biosynthesis of Silver Nanoparticles Using Garcinia Mangostana Fruit Extract and Their Antibacterial, Antioxidant Activity. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 944–952. [Google Scholar]
- Dayanand, M. Fruit Extract: Potential Role in Catalytic Degradation of Methylene. IJPSR 2022, 13, 3187–3193. [Google Scholar]
- Chouhan, S.; Guleria, S. Green Synthesis of AgNPs Using Cannabis Sativa Leaf Extract: Characterization, Antibacterial, Anti-Yeast and α-Amylase Inhibitory Activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis Prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Yassin, M.T.; Al-Otibi, F.O.; Mostafa, A.A.F.; Al-Askar, A.A. Facile Green Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Origanum Majorana with Potential Bioactivity against Multidrug Resistant Bacterial Strains. Crystals 2022, 12, 603. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, B.; Aziz, T.; Zaib, S.; Mansoor, M.A.; Khan, A.A.; Shahzad, M.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Green Synthesis of Silver Nanoparticles Using the Plant Extract of Acer Oblongifolium and Study of Its Antibacterial and Antiproliferative Activity via Mathematical Approaches. Molecules 2022, 27, 4226. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green Synthesis of Silver Nanoparticles Using Hagenia Abyssinica (Bruce) J.F. Gmel Plant Leaf Extract and Their Antibacterial and Anti-Oxidant Activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Salmiati, S.; Marpongahtun, M.; Salim, M.R.; Lolo, J.A.; Syafiuddin, A. Green Synthesis of Silver Nanoparticles Using Muntingia Calabura Leaf Extract and Evaluation of Antibacterial Activities. Biointerface Res. Appl. Chem. 2020, 10, 6253–6261. [Google Scholar]
- de Fernando, F.L.; Caroline, A.B.; Claudia, A.L.C.; Marta, C.T.D.; Eliana, J.S.-A. Evaluation of Nutritional Composition, Bioactive Compounds and Antimicrobial Activity of Elaeocarpus Serratus Fruit Extract. Afr. J. Food Sci. 2019, 13, 30–37. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chowdhury, A.; Das, J.; Chowdhury, A.; Raihan, S.Z.; Muhit, M.A. Phytochemical Investigation with Assessment of Cytotoxicity and Antibacterial Activities of the Ethanol Extract of Elaeocarpus Serratus. Am. J. Plant Physiol. 2012, 7, 47–52. [Google Scholar] [CrossRef]
- Jayashree, I.; Nadu, T. Jayashree et Al. Evaluation of Antimicrobial Potential of Elaeocarpus Serratus L. IJPSR 2014, 5, 3467–3472. [Google Scholar]
- Manoharan, A.L.; Thamburaj, S.; Muniyandi, K.; Jagadeesan, G.; Sathyanarayanan, S.; Nataraj, G.; Thangaraj, P. Antioxidant and Antimicrobial Investigations of Elaeocarpus tectorius (Lour.) Poir. Fruits against Urinary Tract Infection Pathogens. Biocatal. Agric. Biotechnol. 2019, 20, 101260. [Google Scholar] [CrossRef]
- Geetha, D.H.; Rajeswari, M.; Jayashree, I. Chemical Profiling of Elaeocarpus serratus L. by GC-MS. Asian Pac. J. Trop. Biomed. 2013, 3, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Sircar, B.; Mandal, M.; Mondal, M.A.; Mandal, S. Biosynthesis of Elaeocarpus floribundus Mediated Silver Nanoparticles with Broad Antibacterial Spectrum. Acta Sci. Pharm. Sci. 2017, 1, 59–67. [Google Scholar]
- Kumar, T.S.; Shanmugam, S.; Palvannan, T.; Kumar, V.M.B. Evaluation of Antioxidant Properties of Elaeocarpus ganitrus Roxb. Leaves. Iran. J. Pharm. Res. 2008, 7, 211–215. [Google Scholar]
- Singh, B.; Chopra, A.; Ishar, M.P.S.; Sharma, A.; Raj, T. Pharmacognostic and Antifungal Investigations of Elaeocarpus ganitrus (Rudrakasha). Indian J. Pharm. Sci. 2010, 72, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, L.; Amarasinghe, N.R.; Arundathie, B.G.S.; Rupasinghe, G.K.; Jayatilake, N.H.A.N.; Fujimoto, Y. Antioxidant Flavonol Glycosides from Elaeocarpus serratus and Filicium decipiens. Nat. Prod. Res. 2012, 26, 717–721. [Google Scholar] [CrossRef]
- Doğan Çalhan, S.; Gündoğan, M. Biosynthesis of Silver Nanoparticles Using Onosma sericeum Willd. and Evaluation of Their Catalytic Properties and Antibacterial and Cytotoxic Activity. Turk. J. Chem. 2020, 44, 1587–1600. [Google Scholar] [CrossRef]
- Bodke, M.; Gawai, U.; Patil, A.; Dole, B. Estimation of Accurate Size, Lattice Strain Using Williamson-Hall Models, SSP and TEM of Al Doped ZnO Nanocrystals. Mater. Technol. 2018, 106, 602. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green Synthesis of Silver Nanoparticles from Tectona Grandis Seeds Extract: Characterization and Mechanism of Antimicrobial Action on Different Microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Salvesen, I.; Vadstein, O. Evaluation of Plate Count Methods for Determination of Maximum Specific Growth Rate in Mixed Microbial Communities, and Its Possible Application for Diversity Assessment. J. Appl. Microbiol. 2000, 88, 442–448. [Google Scholar] [CrossRef]
- Arif, R.; Uddin, R. A Review on Recent Developments in the Biosynthesis of Silver Nanoparticles and Its Biomedical Applications. Med. Devices Sens. 2021, 4, e10158. [Google Scholar] [CrossRef]
- Abaid, R.; Malik, M.; Iqbal, M.A.; Malik, M.; Shahwani, Z.; Ali, T.Z.; Morsy, K.; Capangpangan, R.Y.; Alguno, A.C.; Choi, J.R. Biosynthesizing Cassia Fistula Extract-Mediated Silver Nanoparticles for MCF-7 Cell Lines Anti-Cancer Assay. ACS Omega 2023, 8, 17317–17326. [Google Scholar] [CrossRef]
- Malik, M.; Iqbal, M.A.; Malik, M.; Raza, M.A.; Shahid, W.; Choi, J.R.; Pham, P.V. Biosynthesis and Characterizations of Silver Nanoparticles from Annona squamosa Leaf and Fruit Extracts for Size-Dependent Biomedical Applications. Nanomaterials 2022, 12, 616. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; González Sánchez, Z.I.; Tungare, K.; Bhori, M.; Durán-Lara, E.F.; Anbu, P. Moringa oleifera Gum Capped MgO Nanoparticles: Synthesis, Characterization, Cyto- and Ecotoxicity Assessment. Int. J. Biol. Macromol. 2023, 233, 123514. [Google Scholar] [CrossRef]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green Synthesis of Silver Nanoparticles Using Terrestrial Fern (Gleichenia pectinata (Willd.) C. Presl.): Characterization and Antimicrobial Studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef]
- Sarwer, Q.; Amjad, M.S.; Mehmood, A.; Binish, Z.; Mustafa, G.; Farooq, A.; Qaseem, M.F.; Abasi, F.; Pérez de la Lastra, J.M. Green Synthesis and Characterization of Silver Nanoparticles Using Myrsine Africana Leaf Extract for Their Antibacterial, Antioxidant and Phytotoxic Activities. Molecules 2022, 27, 7612. [Google Scholar] [CrossRef]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green Synthesis, Characterization, Antibacterial, Antioxidant and Photocatalytic Activity of Parkia Speciosa Leaves Extract Mediated Silver Nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Ameen, F.; Aabed, K.; Saad, R. In-Silico Predicting as a Tool to Develop Plant-Based Biomedicines and Nanoparticles: Lycium shawii Metabolites. Biomed. Pharmacother. 2022, 150, 113008. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Saha, P.; Ochiai, B. Green Synthesis and Catalytic Activity of Silver Nanoparticles Based on Piper chaba Stem Extracts. Nanomaterials 2020, 10, 1777. [Google Scholar] [CrossRef]
- Balavijayalakshmi, J.; Ramalakshmi, V. Carica Papaya Peel Mediated Synthesis of Silver Nanoparticles and Its Antibacterial Activity against Human Pathogens. J. Appl. Res. Technol. 2017, 15, 413–422. [Google Scholar] [CrossRef]
- Chinnasamy, G.; Chandrasekharan, S.; Bhatnagar, S. Biosynthesis of Silver Nanoparticles from Melia Azedarach: Enhancement of Antibacterial, Wound Healing, Antidiabetic and Antioxidant Activities. Int. J. Nanomed. 2019, 14, 9823–9836. [Google Scholar] [CrossRef]
- Jin, Y.; Li, B.; Saravanakumar, K.; Hu, X.; Mariadoss, A.V.A.; Wang, M.H. Cytotoxic and Antibacterial Activities of Starch Encapsulated Photo-Catalyzed Phytogenic Silver Nanoparticles from Paeonia lactiflora Flowers. J. Nanostructure Chem. 2022, 12, 375–387. [Google Scholar] [CrossRef]
- Anuradha, J.; Abbasi, T.; Abbasi, S.A. An Eco-Friendly Method of Synthesizing Gold Nanoparticles Using an Otherwise Worthless Weed Pistia (Pistia stratiotes L.). J. Adv. Res. 2015, 6, 711–720. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Álvarez-Suarez, J.M. Green Synthesis of Silver Nanoparticles Using Astragalus tribuloides Delile. Root Extract: Characterization, Antioxidant, Antibacterial, and Anti-Inflammatory Activities. Nanomaterials 2020, 10, 2383. [Google Scholar] [CrossRef]
- Shehzad, A.; Qureshi, M.; Jabeen, S.; Ahmad, R.; Alabdalall, A.H.; Aljafary, M.A.; Al-Suhaimi, E. Synthesis, Characterization and Antibacterial Activity of Silver Nanoparticles Using Rhazya stricta. PeerJ 2018, 2018, e6086. [Google Scholar] [CrossRef]
- Prasad, K.S.; Pathak, D.; Patel, A.; Dalwadi, P.; Prasad, R.; Patel, P.; Selvaraj, K. Biogenic Synthesis of Silver Nanoparticles Using Nicotiana tobaccum Leaf Extract and Study of Their Antibacterial Effect. Afr. J. Biotechnol. 2011, 10, 8122–8130. [Google Scholar]
- Singh, A.; Gaud, B.; Jaybhaye, S. Optimization of Synthesis Parameters of Silver Nanoparticles and Its Antimicrobial Activity. Mater. Sci. Energy Technol. 2020, 3, 232–236. [Google Scholar] [CrossRef]
- Bawazeer, S.; Rauf, A.; Shah, S.U.A.; Shawky, A.M.; Al-Awthan, Y.S.; Bahattab, O.S.; Uddin, G.; Sabir, J.; El-Esawi, M.A. Green Synthesis of Silver Nanoparticles Using Tropaeolum majus: Phytochemical Screening and Antibacterial Studies. Green Process. Synth. 2021, 10, 85–94. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Jasuja, N.D.; Gupta, D.K.; Reza, M.; Joshi, S.C. Green Synthesis of AgNPs Stabilized with Biowaste and Their Antimicrobial Activities. Braz. J. Microbiol. 2014, 45, 1325–1332. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green Synthesis and Characterization of Silver Nanoparticles Using Banana Peel Extract and Their Antimicrobial Activity against Representative Microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Velu, M.; Lee, J.H.; Chang, W.S.; Lovanh, N.; Park, Y.J.; Jayanthi, P.; Palanivel, V.; Oh, B.T. Fabrication, Optimization, and Characterization of Noble Silver Nanoparticles from Sugarcane Leaf (Saccharum officinarum) Extract for Antifungal Application. 3 Biotech 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Eya’ane Meva, F.; Segnou, M.L.; Ebongue, C.O.; Ntoumba, A.A.; Kedi, P.B.E.; Deli, V.; Etoh, M.A.; Mpondo, E.M. Spectroscopic Synthetic Optimizations Monitoring of Silver Nanoparticles Formation from Megaphrynium macrostachyum Leaf Extract. Rev. Bras. Farmacogn. 2016, 26, 640–646. [Google Scholar] [CrossRef]
- Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrzab, R. The Effect of Ph on the Size of Silver Nanoparticles Obtained in the Reduction Reaction with Citric and Malic Acids. Materials 2020, 13, 5444. [Google Scholar] [CrossRef]
- Alqadi, M.K.; Abo Noqtah, O.A.; Alzoubi, F.Y.; Alzouby, J.; Aljarrah, K. PH Effect on the Aggregation of Silver Nanoparticles Synthesized by Chemical Reduction. Mater. Sci. Pol. 2014, 32, 107–111. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green Synthesis of Silver Nanoparticles Using Olive Leaf Extract and Its Antibacterial Activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Pimprikar, P.S.; Joshi, S.S.; Kumar, A.R.; Zinjarde, S.S.; Kulkarni, S.K. Influence of Biomass and Gold Salt Concentration on Nanoparticle Synthesis by the Tropical Marine Yeast Yarrowia lipolytica NCIM 3589. Colloids Surfaces B Biointerfaces 2009, 74, 309–316. [Google Scholar] [CrossRef]
- Mothana, R.A.; Hussain, H. Phyto-Extract-Mediated Synthesis of Silver Nanoparticles Using Aqueous Extract of Sanvitalia procumbens, and Characterization, Optimization and Photocatalytic Degradation of Azo Dyes Orange G and Direct Blue-15. Molecules 2021, 26, 6144. [Google Scholar]
- Davidović, S.; Lazić, V.; Vukoje, I.; Papan, J.; Anhrenkiel, S.P.; Dimitrijević, S.; Nedeljković, J.M. Dextran Coated Silver Nanoparticles—Chemical Sensor for Selective Cysteine Detection. Colloids Surf. B Biointerfaces 2017, 160, 184–191. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Mandal, D.; Kumar Dash, S.; Das, B.; Chattopadhyay, S.; Ghosh, T.; Das, D.; Roy, S. Bio-Fabricated Silver Nanoparticles Preferentially Targets Gram Positive Depending on Cell Surface Charge. Biomed. Pharmacother. 2016, 83, 548–558. [Google Scholar] [CrossRef]
- Vega-Baudrit, J.; Gamboa, S.M.; Rojas, E.R.; Martinez, V.V. Synthesis and Characterization of Silver Nanoparticles and Their Application as an Antibacterial Agent. Int. J. Biosens. Bioelectron. 2019, 5, 166–173. [Google Scholar] [CrossRef]
- Yarrappagaari, S.; Gutha, R.; Narayanaswamy, L.; Thopireddy, L.; Benne, L.; Mohiyuddin, S.S.; Vijayakumar, V.; Saddala, R.R. Eco-Friendly Synthesis of Silver Nanoparticles from the Whole Plant of Cleome viscosa and Evaluation of Their Characterization, Antibacterial, Antioxidant and Antidiabetic Properties. Saudi J. Biol. Sci. 2020, 27, 3601–3614. [Google Scholar] [CrossRef]
- Soliman, I.; Gunathilake, C.; Paudel, P.R. Nanoarchitectonics of planar organic electrochemical transistors for biological applications and electrochemical sensors. J. Solid State Electrochem. 2023, 023, 05771-9. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic Synthesis, Characterization of Antibacterial Silver Nanoparticles and Its Cell Cytotoxicity. Arab. J. Chem. 2017, 10, 1107–1117. [Google Scholar] [CrossRef]










| Sample | Average Diameter of Zones of Inhibition (mm) | |||
|---|---|---|---|---|
| Pseudomonas aeruginosa | Escherichia coli | Staphylococcus epidermidis | Staphylococcus aureus | |
| AgNPs | 18.4 ± 0.55 a | 14.4 ± 0.55 a | 10.4 ± 0.55 a | 11.6 ± 0.55 a |
| AgNO3 | 13 ± 0.71 b | 11.2 ± 0.83 b | 7.4 ± 0.49 b | 8.4 ± 0.55 b |
| Streptomycin (control) | 14.8± 1.92 b | 13.8 ± 1.09 b | 12.8 ± 0.84 c | 10.4 ± 1.14 ab |
| Ceylon olive extract | N.D | N.D | N.D | N.D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, K.M.; Gunathilake, C.A.; Yalegama, C.; Samarakoon, U.K.; Fernando, C.A.N.; Weerasinghe, G.; Pamunuwa, G.K.; Soliman, I.; Ghulamullah, N.; Rajapaksha, S.M.; et al. Synthesis of Silver Nanoparticles Using Green Reducing Agent: Ceylon Olive (Elaeocarpus serratus): Characterization and Investigating Their Antimicrobial Properties. J. Compos. Sci. 2024, 8, 43. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs8020043
Fernando KM, Gunathilake CA, Yalegama C, Samarakoon UK, Fernando CAN, Weerasinghe G, Pamunuwa GK, Soliman I, Ghulamullah N, Rajapaksha SM, et al. Synthesis of Silver Nanoparticles Using Green Reducing Agent: Ceylon Olive (Elaeocarpus serratus): Characterization and Investigating Their Antimicrobial Properties. Journal of Composites Science. 2024; 8(2):43. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs8020043
Chicago/Turabian StyleFernando, Kumudu M., Chamila A. Gunathilake, Chandi Yalegama, Upeka K. Samarakoon, Chacrawarthige A. N. Fernando, Gangani Weerasinghe, Geethi K. Pamunuwa, Ibrahim Soliman, Nomi Ghulamullah, Suranga M. Rajapaksha, and et al. 2024. "Synthesis of Silver Nanoparticles Using Green Reducing Agent: Ceylon Olive (Elaeocarpus serratus): Characterization and Investigating Their Antimicrobial Properties" Journal of Composites Science 8, no. 2: 43. https://0-doi-org.brum.beds.ac.uk/10.3390/jcs8020043