Significance of the High Abundance of Pentacyclic Triterpenyl and Hopenyl Acetates in Sphagnum Peat Bogs from Northern Spain
Abstract
:1. Introduction
2. Experimental
2.1. Description of the Sampling Sites
2.2. Analytical Methods
3. Results
3.1. Description of the Profiles
3.2. Triterpenoids in the Major Peat-Forming Plants in the Region
3.3. Higher Plant Triterpenoids in the Peat Extracts
3.4. Hopanoid Derivatives in the Peat Extracts
3.5. δ13C Values of Specific Hydrocarbons
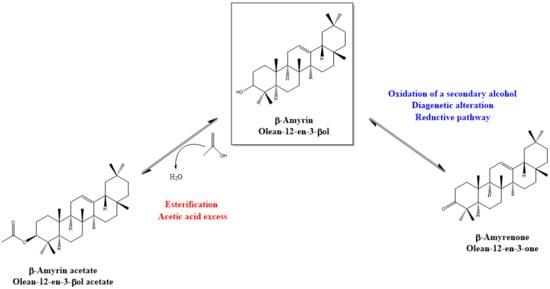
4. Discussion: Relative Abundance of Acetate vs. Ketone Triterpenoids
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mathews, E.; Fung, I. Methane emissions from natural wetlands: Global distribution, area, and environmental characteristics of sources. Glob. Biogeochem. Cycles 1987, 1, 61–86. [Google Scholar] [CrossRef]
- Harriss, R.; Bartlett, K.; Frolking, S.; Crill, P. Methane emissions from northern high-latitude wetlands. In Biogeochemistry of Global Change. Radiatively Active Trace Gases; Oremland, R.S., Ed.; Chapman & Hall: New York, NY, USA, 1993; pp. 449–486. [Google Scholar]
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.G.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the carbon cycle: From local processes to global implications—A synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Jin, Q.; Bohannan, B.; Keller, J.K.; Bridgham, S.D. Homoacetogenesis: A potentially underappreciated carbon pathway in peatlands. Soil Biol. Biochem. 2014, 68, 385–391. [Google Scholar] [CrossRef]
- Krohn, J.; Lozanovska, I.; Kuzyakov, Y.; Parvin, S.; Dorodnikov, M. CH4 and CO2 production below two contrasting peatland micro-relief forms: An inhibitor and δ13C study. Sci. Total Environ. 2017, 586, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.; Simon, B.M. Interaction of acetogens and methanogens in anaerobic fresh water sediments. Appl. Environ. Microbiol. 1985, 49, 944–948. [Google Scholar] [PubMed]
- Zinder, S.H. Physiological ecology of methanogens. In Methanogenesis. Ecology, Physiology, Biochemistry and Genetics; Ferry, J.G., Ed.; Chapman & Hall: New York, NY, USA, 1993; pp. 128–206. [Google Scholar]
- Conrad, R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.R.; Chin, K.-J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.T.; Crawford, R.L. Methane production in Minnesota peatlands. Appl. Environ. Microbiol. 1984, 47, 1266–1271. [Google Scholar]
- Nakagama, F.; Yoshida, N.; Nojiri, Y.; Makarov, V.N. Production of methane from alasses in Eastern Siberia: Implications from its 14C and stable isotopic compositions. Glob. Biogeochem. Cycles 2002, 1041, 14-1–14-15. [Google Scholar]
- Chasar, L.S.; Chanton, J.P.; Glaser, P.H.; Siegel, D.I.; Rivers, J.S. Radiocarbon and stable carbon isotopic evidence for transport and transformation of dissolved organic carbon, dissolved inorganic carbon and CH4 in a northern Minnesota peatland. Glob. Biogeochem. Cycles 2000, 14, 1095–1108. [Google Scholar] [CrossRef]
- Duddleston, K.N.; Kinney, M.A.; Kiene, R.P.; Hines, M.E. Anaerobic microbial biogeochemistry in a northern bog: Acetate as a dominant metabolic end product. Glob. Biogeochem. Cycles 2002, 16, 1063. [Google Scholar] [CrossRef]
- Hines, M.E.; Duddleston, K.N.; Rooney-Varga, J.N.; Fields, D.; Chanton, J.P. Uncoupling of acetate degradation from methane formation in Alaskan wetlands: Connections to vegetation distribution. Glob. Biogeochem. Cycles 2008, 22, 1–12. [Google Scholar] [CrossRef]
- Horn, M.A.; Matthies, C.; Küsel, K.; Schramm, A.; Drake, H.L. Hydrogenotrophic Methanogenesis by Moderately Acid-Tolerant Methanogens of a Methane-Emitting Acidic Peat. Appl. Environ. Microbiol. 2003, 69, 74–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, M.E.; Duddleston, K.N.; Kiene, R.P. Carbon flow to acetate and C1 compounds in northern wetlands. Geophys. Res. Lett. 2001, 28, 4251–4254. [Google Scholar] [CrossRef]
- Shannon, R.D.; White, J.R. The effects of spatial and temporal variations in acetate and sulfate on methane cycling in two Michigan peatlands. Limnol. Oceaongr. 1996, 41, 435–443. [Google Scholar] [CrossRef]
- Keller, J.K.; Bridgham, S.D. Pathways of anaerobic carbon cycling across an ombrotrophic-minerotrophic peatland gradient. Limnol. Oceanogr. 2007, 52, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Jin, Q.; Bohannan, B.; Keller, J.K.; McAllister, S.A.; Bridgham, S.D. pH controls over anaerobic carbon mineralization, the efficiency of methane production, and methanogenic pathways in peatlands across an ombrotrophic-minerotrophic gradient. Soil Biol. Biochem. 2012, 54, 36–47. [Google Scholar] [CrossRef]
- López-Días, V.; Borrego, Á.G.; Blanco, C.G.; Arboleya, M.; López-Sáez, J.A.; López-Merino, L. Biomarkers in a peat deposit in Northern Spain (Huelga de Bayas, Asturias) as proxy for climate variation. J. Chromatogr. A 2010, 1217, 3538–3546. [Google Scholar] [CrossRef]
- López-Días, V.; Borrego, A.G.; Blanco, C.G. Vertical evolution of petrographic and organic geochemical parameters in Las Dueñas mire (Cantabrian Coast, North Spain). Int. J. Coal Geol. 2010, 84, 179–189. [Google Scholar] [CrossRef]
- López-Días, V.; Urbanczyk, J.; Blanco, C.G.; Borrego, A.G. Biomarkers as palaeoclimate proxies in peatlands in coastal high plains in Asturias, N Spain. Int. J. Coal Geol. 2013, 116–117, 270–280. [Google Scholar] [CrossRef]
- López-Dias, V. Geoquímica orgánica y evolución ambiental de turberas de las rasas costeras asturianas. Ph.D. Thesis, University of Oviedo, Oviedo, Spain, 2013; 286p. [Google Scholar]
- Cox, R.E.; Maxwell, J.R.; Ackman, R.G.; Hooper, S.N. The isolation of a series of acyclic alcohols from an ancient sediment: Approaches to a study of the diagenesis and maturation of phytol. In Advances in Organic Geochemistry 1971; von Gaertner, H.R., Wehner, H., Eds.; Pergamon: Oxford, UK, 1972; pp. 263–276. [Google Scholar]
- Zocatelli, R.; Jacob, J.; Gogo, S.; Milbeau, C.L.; Laggoun-Défarge, F. Spatial variability of soil lipids reflects vegetation cover in a French peatland. Org. Geochem. 2014, 76, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Ohmoto, T.; Ikuse, M.; Natori, S. Triterpenoids of the Gramineae. Phytochemistry 1970, 9, 2137–2148. [Google Scholar] [CrossRef]
- Jacob, J.; Disnar, J.R.; Boussafir, M.; Sifeddine, A.; Albuquerque, A.L.S.; Turcq, B. Pentacyclic triterpene methyl ethers in recent lacustrine sediments (Lagoa do Caçó, Brazil). Org. Geochem. 2005, 36, 449–461. [Google Scholar] [CrossRef]
- Zocatelli, R.; Jacob, J.; Turcq, B.; Boussafir, M.; Sifeddine, A.; Bernardes, M.C. Molecular evidence for recent turf cultivation in Northeast Brazil (Lagoa do Boqueirão, RN State). Org. Geochem. 2010, 41, 427–430. [Google Scholar] [CrossRef]
- Luger, P.; Weber, M.; Dung, N.X.; Ngoc, P.H.; Tuong, D.T.; Rang, D.D. The crystal structure of Hop-17(21)-en-3-yl acetate of Pluchea pteropoda Hemsl. from Vietnam. Cryst. Resolut. Technol. 2000, 35, 355–362. [Google Scholar] [CrossRef]
- Lavrieux, M.; Jacob, J.; Le Milbeau, C.; Zocatelli, R.; Masuda, K.; Bréheret, J.G.; Disnar, J.R. Occurrence of triterpenyl acetates in soil and their potential as hemotaxonomical markers of Asteraceae. Org. Geochem. 2011, 42, 1315–1323. [Google Scholar] [CrossRef]
- Weinstein Teixeira, E.; Message, D.; Negri, G.; Salatino, A. Bauer-7-en-3-yl acetate: A major constituent of unusual samples of Brazilian propolis. Química Nova 2006, 29, 245–246. [Google Scholar] [CrossRef]
- Bracho, J.C.; Rodriguez, C.; Llanes, F. Triterpenos pentacíclicos en propóleo. Rev. Soc. Quím. Perú 2009, 75, 439–452. [Google Scholar]
- Flor, G. Las rasas asturianas: Ensayo de correlación y emplazamiento. In Trabajos de Geología; Universidad de Oviedo: Oviedo, Spain, 1983; Volume 13, pp. 65–81. [Google Scholar]
- Menéndez Amor, J. Estudio de las turberas de la zona oriental asturiana. Asoc. Esp. Prog. Cienc. 1950, 15, 801–816. [Google Scholar]
- Fernández Prieto, J.A.; Fernández Ordóñez, M.C.; Collado Prieto, M.A. Datos sobre la vegetación de las “turberas de esfagnos” galaico-asturianas y orocantábricas. Lazaroa 1987, 7, 443–447. [Google Scholar]
- Borrego, A.G.; López-Días, V.; Urbancyk, J.; Díaz, T.E.; Fernandez Casado, M.A.; Fernandez Ordoñez, C.; Gutierrez, I.; Homet, J.; Arboleya, M.; Blanco, C.G. Relationship between the vegetation and the biomarkers and Palynological assemblages in Asturian mires (N spain). In Proceedings of the Organic Geochemistry: Trends for the 21st Century, IMOG 2013, Tenerife, Spain, 15–20 September 2013; Volume I, pp. 318–319. [Google Scholar]
- López-Días, V.; Urbanczyk, J.; Blanco, C.G.; Borrego, A.G. Maceral composition and molecular markers of two condensed Middle Holocene peat profiles in N Spain. Int. J. Coal Geol. 2016, 168 Pt 1, 55–70. [Google Scholar] [CrossRef]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of Triterpenoids Saponins in Plants. Adv. Biochem. Eng./Biotechnol. 2002, 75, 31–49. [Google Scholar]
- Cranwell, P.A. Organic geochemistry of lacustrine sediments: Triterpenoids of higher plants origin reflecting post-glacial vegetational succession. In Lakes Sediments and Environmental History; Haworth, E.Y., Lund, J.W.G., Eds.; University Press: Leicester, UK, 1984; pp. 69–92. [Google Scholar]
- Simoneit, B.R.T. Cyclic terpenoids of the geosphere. In Biological Markers in the Sedimentary Record; Johns, R.B., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 175–221. [Google Scholar]
- Karrer, W.; Hürlimann, H.; Cherbuliez, E. Konstitution und Vorkommen der organischen Pflanzenstoffe, Ergänzungsband 2 (1); Birkhäuser: Basel, Switzerland; Stuttgart, Germany, 1981. [Google Scholar]
- Logan, G.A.; Eglinton, G. Biogeochemistry of the Miocene lacustrine deposit at Clarkia, northern Idaho, USA. Org. Geochem. 1994, 21, 857–870. [Google Scholar] [CrossRef]
- Jaffé, R.; Elimé, T.; Cabrera, A.C. Organic geochemistry of seasonally flooded rainforest soils: Molecular composition and early diagenetic of lipid components. Org. Geochem. 1996, 25, 9–17. [Google Scholar] [CrossRef]
- Hanisch, S.; Ariztegui, D.; Püttmann, W. The biomarker record of Lake Albano, central Italy—Implications for Holocene aquatic system response to environmental change. Org. Geochem. 2003, 34, 1223–1235. [Google Scholar] [CrossRef]
- Baas, M.; Pancost, R.; van Geel, B.; Sinninghe Damsté, J.S. A comparative study of lipids in Sphagnum species. Org. Geochem. 2000, 31, 535–541. [Google Scholar] [CrossRef]
- ten Haven, H.L.; Peakman, T.M.; Rullkotter, J. D2-Triterpenes: Early intermediates in the diagenesis of terrigenous triterpenoids. Geochim. Cosmochim. Acta 1992, 56, 1993–2000. [Google Scholar] [CrossRef]
- Pancost, R.D.; Baas, M.; van Geel, B.; Sinninghe Damsté, J.S. Biomarkers as proxies for plant inputs to peats: An example from a sub-boreal ombrotrophic bog. Org. Geochem. 2002, 33, 675–690. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Díaz-Bautista, A.; Aldasoro, J.J.; Torres, T.; Gallego, J.L.R.; Moreno, L.; Estébanez, B. n-Alkan-2-ones in peat-forming plants from the Roñanzas ombrotrophic bog (Asturias, northern Spain). Org. Geochem. 2011, 42, 586–592. [Google Scholar] [CrossRef]
- Arboleya, M. Biomarcadores moleculares como indicadores paleoclimáticos en turberas asturianas de ambiente submontano. Master’s Thesis, University of Oviedo, Oviedo, Spain, 2011; 30p. [Google Scholar]
- López-Días, V.; López Sáez, J.A.; Dorado Valiño, M.; Püttmann, W.; Fiebig, J.; Blanco, C.G.; Borrego, A.G. Integrating palynological and organic geochemical proxies for the palaeoenvironmental study of the Roñanzas peatbog (N Spain). IX European Wetland Congress: 6°. In Proceedings of the European Pond Conservation Network, Huesca, Spain, 14–18 September 2014. [Google Scholar]
- López-Días, V.; Püttmann, W.; Fiebig, J.; Blanco, C.G.; Borrego, A.G. Palaeoenvironmental implications of the biomarkers distribution in a 6000 years BP peat bog in N Spain. In Proceedings of the Organic Geochemistry: Trends for the 21st Century, IMOG 2013, Tenerife, Spain, 15–20 September 2013; Volume II, pp. 277–278. [Google Scholar]
- Ortiz, J.E.; Gallego, J.L.R.; Torres, T.; Díaz-Bautista, A.; Sierra, C. Palaeoenvironmental recontruction of Northern Spain during the last 8000 cal yr BP based on biomarker content of the Roñanzas peat bog (Asturias). Org. Geochem. 2010, 41, 454–466. [Google Scholar] [CrossRef]
- Ourisson, G.; Rohmer, M.; Poralla, K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu. Rev. Microbiol. 1987, 62, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Belin, B.J.; Busset, N.; Giraud, E.; Molinaro, A.; Silipo, A.; Newman, D.K. Hopanoid lipids: From membranes to plant-bacteria interactions. Nat. Rev. Microbiol. 2018, 16, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Moldowan, J. Effects of source, thermal maturity, and biodegradation on the distribution and isomerization of homohopanes in petroleum. Org. Geochem. 1991, 17, 47–61. [Google Scholar] [CrossRef]
- Quirk, M.M.; Wardroper, A.M.K.; Weatley, R.E.; Maxwell, J.R. Extended hopanoids in peat. Chem. Geol. 1984, 42, 25–43. [Google Scholar] [CrossRef]
- Rosa-Putra, S.; Nalin, R.; Domenach, A.M.; Rohmer, M. Novel hopanoids from Frankia spp. and related soil bacteria. Squalene cyclization and significance of geological biomarkers revisited. Eur. J. Biochem. 2001, 268, 4300–4306. [Google Scholar] [CrossRef] [PubMed]
- van Dorsselaer, A.; Albrecht, P.; Connan, J. Changes in composition of polycyclic alkanes by thermal maturation (Yallourn Lignite, Australia). In Advances in Organic Geochemistry; Campos, R., Goñi, J., Eds.; Enadimsa: Madrid, Spain, 1977; pp. 53–59. [Google Scholar]
- Ries-Kautt, M.; Albrecht, P. Hopane derived triterpenoids in soils. Chem. Geol. 1989, 76, 143–151. [Google Scholar] [CrossRef]
- Inglis, G.N.; David, B.; Naafs, A.; Zheng, Y.; McClymont, E.L.; Evershed, R.P.; Pancost, R.D.; the ‘T-GRES Peat Database collaborators. Distributions of geohopanoids in peat: Implications for the use of hopanoid-based proxies in natural archives. Geochim. Cosmochim. Acta 2018, 224, 249–261. [Google Scholar] [CrossRef]
- Ageta, H.; Arai, Y. Fern constituents: Pentacyclic triterpenoids isolated from Polypodium niponicum and P. formosanum. Phytochemistry 1983, 22, 1801–1808. [Google Scholar] [CrossRef]
- Toyota, M.; Masuda, K.; Asakawa, Y. Triterpenoid constituents of the moss Floribundaria aurea subsp. Nipponia. Phytochemistry 1998, 48, 297–299. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Xue, J.; Meyers, P.H.; Zhang, Z.; Tan, K.; Zhang, Z.; Xie, S. Occurrence of diploptene in moss species from the Dajiuhu peatland in southern China. Org. Geochem. 2010, 41, 321–324. [Google Scholar] [CrossRef]
- Kannenberg, E.L.; Poralla, K. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 1999, 86, 168–176. [Google Scholar] [CrossRef]
- Fazakerley, H.; Halsall, T.G.; Jones, E.R.H. The chemistry of triterpenes and related compounds. Part XXXIV. The structure of Hydroxihopanone. J. Chem. Soc. 1959, 1877–1883. [Google Scholar] [CrossRef]
- Mitova, M.; Taskova, R.; Popov, S.; Berger, R.G.; Krings, U.; Handjieva, N. GC/MS Analysis of Some Bioactive Constituents from Cart. Lanatus L. Z. Nat. 2003, 58c, 697–703. [Google Scholar]
- Fischer, W.W.; Pearson, A. Hypotheses for the origin and early evolution of triterpenoid cyclases. Geobiology 2007, 5, 19–34. [Google Scholar] [CrossRef]
- Koops, A.J.; Baas, W.J.; Groeneveld, H.W. The composition of phytosterols, latex triterpenols and wax triterpenoids in the seedling of Euphorbia lathyris L. Plant Sci. 1991, 74, 185–191. [Google Scholar] [CrossRef]
- David, J.P.; Meira, M.; David, J.M.; Guedes ML da, S. Triterpenos e ferulatos de alquila de Maprounea guianensis. Quim. Nova 2004, 27, 62–65. [Google Scholar] [CrossRef]
- Kakuda, R.; Iijima, T.; Yaoita, Y.; Machida, K.; Kikuchi, M. Triterpenoids from Gentiana scabra. Phytochemistry 2002, 59, 791–794. [Google Scholar] [CrossRef]
- Hui, W.-H.; Li, M.-M. Six new triterpenoids and other triterpenoids and steroids from three Quercus species of Hong Kong. J. Chem. Soc. Perkin Trans. I 1977, 897–904. [Google Scholar] [CrossRef]
- Arthur, H.R.; Hui, W.H.; Lam, C.N.; Szeto, S.K. An examination of Quercus championi of Hong Kong. Aust. J. Chem. 1964, 17, 697–700. [Google Scholar] [CrossRef]
- Rowan, D.D.; Russell, G.B. 3/3-Methoxyhop-22(29)-ene from Chionochloa Cheesemanii. Phytochemistry 1992, 31, 702–703. [Google Scholar] [CrossRef]
- Pancost, R.D.; Baas, M.; van Geel, B.; Sinninghe Damsté, J.S. Response of an ombrotrophic bog to a regional climate event revealed by macrofossil, molecular and carbon isotopic data. Holocene 2003, 13, 921–932. [Google Scholar] [CrossRef]
- Xie, S.; Nott, C.J.; Avsejs, L.A.; Maddy, D.; Chambers, F.M.; Evershed, R.P. Molecular and isotopic stratigraphy in an ombrotrophic mire for paleoclimate reconstruction. Geochim. Cosmochim. Acta 2004, 13–68, 2849–2862. [Google Scholar] [CrossRef]
- Naafs, B.D.A.; Inglis, G.N.; Blewetta, J.; McClymontc, E.L.; Lauretano, V.; Xie, S.; Evershed, R.P.; Pancost, R.D. The potential of biomarker proxies to trace climate, vegetation, and biogeochemical processes in peat: A review. Glob. Planet. Chang. 2019, 179, 57–79. [Google Scholar] [CrossRef]
- Hayes, J.M. Factors controlling 13C contents of sedimentary organic compounds: Principles and evidence. Mar. Geol. 1993, 113, 111–125. [Google Scholar] [CrossRef]
- van Winden, J.F.; Kip, N.; Reichart, G.-J.; Jetten, M.S.M.; Op den Camp, H.J.M.; Damsté, J.S.S. Lipids of symbiotic methane-oxidizing bacteria in peat moss studied using stable carbon isotopic labelling. Org. Geochem. 2010, 41, 1040–1044. [Google Scholar] [CrossRef]
- Huang, X.; Pancost, R.D.; Xue, J.; Gu, Y.; Evershed, R.P.; Xie, S. Response of carbon cycle to drier conditions in the mid-Holocene in central China. Nat. Commun. 2018, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kawamura, K.; Seki, O.; Meyers, P.A.; Zheng, Y.; Zhou, W. Environmental influences over the last 16 ka on compound-specific 13C variations of leaf wax n-alkanes in the Hani peat deposit from northeast China. Chem. Geol. 2010, 277, 261–268. [Google Scholar] [CrossRef]
- López-Días, V.; Blanco, C.G.; Bechtel, A.W.; Püttmann, W.; Borrego, A.G. Different source of n-alkanes and n-alkane-2-ones in a 6000 cal. yr BP Sphagnum-rich temperate peat bog (Roñanzas, N Spain). Org. Geochem. 2013, 57, 7–10. [Google Scholar] [CrossRef]
- Urbanczyk, J.; Bechtel, A.; Borrego, A.G. Organic geochemical evidence of postglacial paleoenvironmental evolution of the Comeya peatland (Asturias, N Spain). Int. J. Coal Geol. 2017, 168, 46–54. [Google Scholar] [CrossRef]
- Huang, Y.S.; Bol, R.; Harkness, D.D.; Ineson, P.; Eglinton, G. Post-glacial variations in distributions,13C and 14C contents of aliphatic hydrocarbons and bulk organic matter in three types of British acid upland soils. Org. Geochem. 1996, 24, 273–287. [Google Scholar] [CrossRef]
- Pancost, R.D.; van Geel, B.; Baas, M.; Sinninghe Damsté, J.S. 13C values and radiocarbon dates of microbial biomarkers as tracers for carbon recycling in peat deposits. Geology 2000, 28, 1126–1132. [Google Scholar] [CrossRef]
- Kool, D.M.; Talbot, H.M.; Rush, D.; Ettwig, K.; Damste, J.S.S. Rare bacteriohopanepolyols as markers for an autotrophic, intra-aerobic methanotroph. Geochim. Cosmochim. Acta 2014, 136, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Trendel, J.M.; Schaeffer, P.; Adam, P.; Ertlen, D.; Schwartz, D. Molecular characterisation of soil surface horizons with different vegetation in the Vosges Massif (France). Org. Geochem. 2010, 41, 1036–1039. [Google Scholar] [CrossRef]
- Bull, I.D.; van Bergen, P.F.; Nott, C.J.; Poulton, P.R.; Evershed, R.P. Organic geochemical studies of soils from the Rothamsted classical experiments. V. The fate of lipids in different long-term experiments. Org. Geochem. 2000, 31, 389–408. [Google Scholar] [CrossRef]
- van Bergen, P.; Bull, I.; Poulton, P.R.; Evershed, R.P. Organic geochemical studies of soils from the Rothamsted Classical Experiments—1. Total lipid extracts, solvent insoluble residues and humic acids from Broadbalk Wilderness. Org. Geochem. 1997, 26, 117–135. [Google Scholar] [CrossRef]
- Oyo-Ita, O.E.; Ekpo, B.O.; Oros, D.R.; Simoneit, B.R.T. Occurrence and sources of triterpenoid methyl ethers and acetates in sediments of the Cross-River system, Southeast Nigeria. Int. J. Anal. Chem. 2010, 2010, 502076. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatari, R. Lacustrine organic geochemistry—An overview of indicators of organic matter sources and diagenesis in lake sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Drake, H.L.; Horn, M.A.; Wust, P.K. Intermediary ecosystem metabolism as a main driver of methanogenesis in acidic wetland soil. Environ. Microbiol. Rep. 2009, 1, 307–318. [Google Scholar] [CrossRef]
- Chauhan, A.; Ogram, A. Phylogeny of Acetate-Utilizing Microorganisms in Soils along a Nutrient Gradient in the Florida Everglades. Appl. Environ. Microbiol. 2006, 72, 6837–6840. [Google Scholar] [CrossRef] [Green Version]
- Cadillo-Quiroz, H.; Yavitt, J.; Zinder, S.; Thies, J. Diversity and community structure of Archaea inhabiting the rhizoplane of two contrasting plants from an acidic bog. Microb. Ecol. 2010, 59, 757–767. [Google Scholar] [CrossRef] [PubMed]









| Compound | C. Vulgaris | E. Mackaiana | M. Caerulea | S. Cuspidatum | S. Denticulatum | S. Papillosum | S. Tenellum |
|---|---|---|---|---|---|---|---|
| Ergost-5-en-3β-ol (Campesterol) | 0.09 | 69.70 | 0.39 | 0.53 | 0.51 | ||
| Stigmast-5, 22-en-3β-ol (Stigmasterol) | 177.56 | 6.41 | 3.49 | 1.49 | |||
| γ-Sitosterol (Stigmast-5-en-3β-ol) | 1.12 | 18.49 | 0.79 | 0.30 | 0.22 | ||
| Ergost-4-en-3-one | 0.08 | 0.28 | |||||
| 5α-Stigmastanone | 0.10 | ||||||
| 24-Etil-cholestan-3, 5-dien-7-one | 0.06 | 0.70 | 0.07 | ||||
| Stigmast-4-en-3-one | 5.18 | 1.24 | 0.42 | 0.22 | |||
| β-Amyrin | 55.65 | 110.41 | 4.07 | 0.14 | 2.80 | 0.45 | |
| β-Amyrenone (olean-12-en-3-one) | 0.13 | ||||||
| β-Amyrin Acetate (olean-12-en-3b-ol Acetate) | 4.64 | 1.49 | 0.22 | ||||
| α-Amyrin | 135.30 | 233.82 | 9.00 | 6.29 | 1.35 | ||
| α-Amyrenone (urs-12-en-3-one) | 1.82 | 0.63 | 0.22 | ||||
| α-Amyrin Acetate (Urs-12-en-3b-ol Acetate) | 9.12 | 8.60 | 0.23 | ||||
| Friedelin (D:A-Friedooleanan-3-one) | 0.68 | ||||||
| Lup-20 (29)-en-3-one | 0.16 | 0.01 | 0.08 | 0.02 | |||
| Lup-20(29)-en-3β-ol Acetate | 6.42 | ||||||
| hop-22 (29) en-3-ol | 0.07 | ||||||
| hop-22 (29) en-3-one | 0.04 |
| Depth | C31 αβ R-Homopane | 22, 29, 30-Trisnorhopan-21-one | Hop-17(21)-en-3-one | Hop-17(21)-en-3β-ol Acetate | Hop-22(29)en-3-one | Hop-22(29)en-3β-ol Acetate | β-Amyrenone (olean-12-en-3-one) | β-Amyrin Acetate (olean-12-en-3β-ol Acetate) | Friedelin (D:A-Friedooleanan-3-one) | Friedelinol Acetate (D:A-Friedooleanan-3β-ol Acetate | α-Amyrenone (urs-12-en-3-one) | α-Amyrin Acetate (Urs-12-en-3β-ol Acetate) | Lupan-3-one | Lup-20(29)-en-3β-ol Acetate) | D:C-Friedours-7-en-3-one (Bauerenone) | D:C-Friedours-7-en-3β-ol Acetate (Bauer-7-en-3β-ol Acetate) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.5 | 0.6 | 16.7 | 10.0 | 3.3 | 1.0 | 0.1 | 1.3 | 0.5 | ||||||||
| 9.5 | 0.4 | 34.6 | 17.2 | 11.9 | 2.3 | 38.8 | 0.8 | 0.5 | 0.6 | |||||||
| 13 | 2. 7 | 1.7 | 1.6 | 3.6 | 0.8 | 17.5 | 0.8 | 2.8 | ||||||||
| 19 | 70.6 | 18.5 | 64.0 | 25.5 | 15.3 | 1.9 | 17.1 | 1.6 | 2.7 | |||||||
| 23 | 39.1 | 71.8 | 27.5 | 13.5 | 3.5 | 0.8 | 7.3 | 10.5 | ||||||||
| 27 | 5.5 | 9.0 | 28.1 | 12.0 | 2.0 | 0.5 | ||||||||||
| 33 | 420.1 | 62.6 | 67.2 | 50.9 | 8.4 | 2.7 | 14.3 | 5.1 | 3.0 | 40.0 | ||||||
| 39 | 168.7 | 3.7 | 102.4 | 66.8 | 13.8 | 4.6 | 5.2 | 83.9 | 8.3 | 8.4 | 8.5 | 11.8 | 63.7 | |||
| 43 | 269.4 | 3.3 | 15.8 | 16.9 | 6.3 | 2.9 | 2.9 | 26.5 | 15.4 | 11.2 | 6.3 | 18.9 | 78.0 | |||
| 47 | 84.2 | 0.5 | 2.0 | 2.0 | 0.2 | 2.1 | 1.5 | 2.3 | 1.2 | 1.2 | 2.2 | 12.9 | ||||
| 53 | 376.9 | 2.5 | 27.1 | 13.3 | 0.8 | 0.5 | 5.3 | 4.0 | 3.6 | 2.9 | 5.6 | 24.4 | ||||
| 57 | 127.5 | 0.5 | 1.2 | 1.5 | 2.5 | 4.6 | 2.4 | 1.1 | 1.7 | 2.9 | 15.5 | |||||
| 63 | 207.1 | 0.7 | 6.3 | 1.2 | 1.3 | 2.3 | 5.9 | 2.7 | 7.2 | 3.2 | 1.9 | 7.7 | 33.7 | |||
| 67 | 258.0 | 0.9 | 55.9 | 14.3 | 12.6 | 1.5 | 3.2 | 6.3 | 5.8 | 3.9 | ||||||
| 73 | 392.3 | 3.5 | 28.8 | 16.4 | 10.4 | 1.6 | 10.5 | 12.3 | 13.5 | 2.2 | 14.8 | 7.7 | 85.5 | |||
| 79 | 33.7 | 1.6 | 47.8 | 41.7 | 9.4 | 4.8 | 3.9 | 11.3 | 3.9 | 5.6 | 6.1 | 112.9 | ||||
| 83 | 12.8 | 2.5 | 69.0 | 19.4 | 9.7 | 1.4 | 1.2 | 3.5 | 0.6 | 16.3 | ||||||
| 87 | 37.7 | 3.5 | 76.5 | 31.9 | 13.6 | 4.5 | 3.3 | 72.8 | 3.8 | 1.2 | 15.5 | |||||
| 93 | 11.2 | 9.8 | 39.7 | 23.8 | 5.8 | 1.7 | 2.2 | 48.7 | 1.8 | 1.8 | 0.2 | 3.3 | ||||
| 97 | 32.9 | 1.8 | 5.4 | 2.7 | 1.2 | 0.2 | 3.5 | 67.3 | 0.7 | |||||||
| 103 | 171.3 | 0.9 | 1.1 | 4.9 | 9.3 | 5.4 | 1.1 | 3.4 | 1.6 | 30.3 | ||||||
| 107 | 111.3 | 1.5 | 1.3 | 17.4 | 2.8 | 1.3 | 0.5 | 6.3 | ||||||||
| 113 | 52.7 | 2.9 | 2.3 | 1.6 | 0.6 | 3.7 | 15.1 | 22.1 | 9.5 | 2.7 | 4.9 | 6.0 | 48.7 | |||
| 117 | 52.7 | 2.1 | 4.7 | 17.0 | 3.7 | 1.5 | 3.9 | 20.4 | ||||||||
| 123 | 55.8 | 0.4 | 2.8 | 4.1 | 15.3 | 3.9 | 1.1 | 1.1 | 6.3 | 18.2 | ||||||
| 127 | 54.6 | 0.4 | 8.0 | 11.4 | 40.3 | 8.9 | 2.7 | 6.3 | 7.7 | 45.8 | ||||||
| 133 | 18.1 | 0.3 | 2.6 | 9.0 | 11.5 | 6.4 | 1.2 | 2.4 | 6.4 | 44.3 | ||||||
| 137 | 21.0 | 1.5 | 8.3 | |||||||||||||
| 143 | 61.4 | 0.8 | 13.9 | 3.1 | 60.0 | 5.7 | 2.6 | 1.2 | 5.9 | 12.3 | ||||||
| 147 | 67.1 | 0.6 | 10.2 | 21.7 | 19.2 | 39.9 | 8.2 | 10.9 | 24.1 | 85.8 | ||||||
| 153 | 32.1 | 0.3 | 9.0 | 18.9 | 14.6 | 21.5 | 5.5 | 7.6 | 28.7 | 88.1 | ||||||
| 157 | 36.8 | 0.6 | 15.7 | 28.7 | 23.0 | 23.1 | 12.1 | 11.3 | 53.7 | 127.4 | ||||||
| 163 | 23.0 | 0.3 | 9.0 | 14.7 | 15.5 | 13.4 | 8.3 | 7.7 | 25.2 | 68.6 | ||||||
| 167 | 88.3 | 0.6 | 26.7 | 31.3 | 35.7 | 24.3 | 24.8 | 9.4 | 77.9 | 97.1 | ||||||
| 173 | 80.2 | 0.8 | 3.2 | 2.6 | 4.5 | 3.5 | 2.3 | 0.8 | 7.6 | 7.1 | ||||||
| 177 | 41.9 | 0.9 | 15.6 | 22.8 | 17.1 | 16.4 | 14.6 | 11.1 | 38.1 | 69.1 | ||||||
| 183 | 33.9 | 0.2 | 8.4 | 4.6 | 38.2 | 3.3 | 2.9 | 1.7 | 9.4 | 18.5 | ||||||
| 187 | 30.8 | 0.1 | 2.0 | 1.0 | 3.3 | 0.7 | 0.8 | 0.2 | 2.8 | 3.9 | ||||||
| 193 | 41.7 | 0.2 | 7.3 | 11.5 | 6.7 | 15.3 | 8.8 | 6.4 | 22.2 | 36.6 | ||||||
| 197 | 70.4 | 0.4 | 7.1 | 5.3 | 10.6 | 5.8 | 4.4 | 1.5 | 23.6 | 28.5 | ||||||
| 203 | 114.1 | 26.0 | 43.3 | 32.7 | 42.2 | 26.9 | 23.5 | 67.6 | 117.2 | 4.1 | ||||||
| 207 | 21.9 | 0.3 | 2.0 | 2.4 | 2.6 | 2.3 | 1.5 | 0.7 | 4.7 | 6.6 | 1.4 | |||||
| 213 | 10.9 | 0.3 | 0.6 | 0.3 | 1.1 | 0.6 | 0.4 | 0.1 | 1.5 | 1.0 | ||||||
| 217 | 7.0 | 0.3 | 0.6 | 0.5 | 0.6 | 0.4 | ||||||||||
| 223 | 34.8 | 0.3 | 0.6 | 1.2 | 0.3 | 12.4 | ||||||||||
| 227 | 20.2 | 0.3 | 0.8 | 1.0 | 0.8 | 0.9 | 0.4 | 1.6 | 3.2 | 1.4 | 15.2 | |||||
| 233 | 12.3 | 0.2 | 0.3 | 0.2 | 0.4 | 0.5 | 0.8 | 9.4 | ||||||||
| 237 | 3.1 | 0.3 | 0.2 | 1.3 | 0.4 | 0.2 | 0.3 | 2.0 | 0.5 | 1.4 | 3.3 | 12.2 |
| Depth | C31αβ R-Homopane | 22, 29, 30-Trisnorhopan-21-one | Hop-17(21)-en-3-one | Hop-17(21)-en-3β-ol Acetate | Hop-22(29)en-3-one | Hop-22(29)en-3β-ol Acetate | β-Amyrenone (olean-12-en-3-one) | β-Amyrin Acetate (olean-12-en-3β-ol Acetate) | Friedelin (D:A-Friedooleanan-3-one) | Friedelinol Acetate (D:A-Friedooleanan-3β-ol Acetate | α-Amyrenone (urs-12-en-3-one) | α-Amyrin Acetate (Urs-12-en-3β-ol Acetate) | Lupan-3-one | Lup-20(29)-en-3β-ol Acetate) | D:C-Friedours-7-en-3-one (Bauerenone) | D:C-Friedours-7-en-3β-ol Acetate (Bauer-7-en-3β-ol Acetate) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BUELNA | ||||||||||||||||
| 7.5 | 9.6 | 0.7 | 4.6 | 5.5 | 2.2 | 0.8 | 0.7 | 3.1 | ||||||||
| 16 | 13.4 | 1.6 | 20.0 | 12.2 | 3.4 | 1.2 | 0.3 | 2.3 | 1.8 | 0.4 | 0.4 | 1.6 | 4.5 | 0.2 | 12.9 | |
| 20 | 12.4 | 1.7 | 20.6 | 12.6 | 2.8 | 0.9 | 0.5 | 5.0 | 0.7 | 0.5 | 0.4 | 1.1 | 0.6 | 0.5 | 23.6 | |
| 24 | 3.3 | 1.3 | 4.9 | 2.4 | 1.1 | 0.3 | 0.3 | 2.5 | 0.4 | 0.2 | 0.3 | 1.0 | 0.3 | 0.5 | 22.4 | |
| 28 | 0.6 | 0.9 | 0.4 | 0.1 | 0.0 | 0.6 | 5.1 | 0.3 | 0.2 | 2.2 | 0.2 | 5.2 | ||||
| 32 | 0.3 | 0.6 | 0.5 | 2.3 | 0.1 | 0.2 | 2.6 | 0.4 | 13.3 | |||||||
| 36 | 0.1 | 0.2 | 0.1 | 0.1 | 0.6 | 0.1 | 0.0 | 0.2 | 0.6 | 0.1 | 0.3 | 18.4 | ||||
| 40 | 0.3 | 0.6 | 0.5 | 0.1 | 9.2 | 0.1 | 0.4 | 1.9 | 0.7 | 48.8 | ||||||
| 44 | 0.1 | 0.2 | 0.1 | 0.1 | 0.4 | 0.1 | 0.0 | 0.2 | 0.5 | 0.1 | 0.2 | 20.9 | ||||
| 48 | 0.6 | 0.7 | 0.3 | 0.1 | 5.0 | 0.1 | 0.5 | 1.0 | 0.3 | 0.5 | 54.6 | |||||
| 52 | 0.2 | 0.7 | 0.2 | 0.2 | 0.5 | 0.1 | 0.4 | 0.9 | 0.3 | 0.4 | 46.0 | |||||
| 56 | 6.3 | 0.7 | 0.3 | 0.1 | 1.0 | 0.1 | 0.6 | 1.2 | 0.7 | 75.8 | ||||||
| 60 | 3.0 | 2.0 | 0.4 | 0.6 | 1.0 | 1.2 | 0.1 | 1.1 | 2.0 | 0.8 | 1.2 | 105.4 | ||||
| 62 | 0.5 | 0.3 | 0.1 | 0.3 | 0.3 | 0.1 | 7.1 | |||||||||
| 64 | 1.4 | 1.0 | 0.2 | 0.0 | 2.0 | 0.4 | 0.9 | 0.2 | 10.3 | |||||||
| 68 | 0.6 | 0.7 | 0.4 | 0.0 | 11.2 | 1.7 | 1.1 | 0.1 | 1.6 | |||||||
| LABORBOLLA | ||||||||||||||||
| 3.5 | 16.8 | 2.5 | 26.0 | 19.1 | 2.7 | 1.4 | 0.9 | 0.3 | 13.5 | 1.8 | 0.9 | 0.4 | 0.9 | 3.8 | 0.2 | 7.9 |
| 8 | 31.5 | 9.7 | 45.5 | 31.3 | 12.8 | 4.0 | 3.8 | 1.0 | 114.2 | 10.7 | 2.3 | 2.5 | 4.2 | 16.0 | 1.4 | 50.6 |
| 12 | 10.2 | 8.3 | 108.2 | 38.8 | 23.5 | 8.8 | 1.2 | 39.0 | 1.5 | 0.5 | 0.5 | 2.0 | 5.6 | 54.9 | ||
| 16 | 9.5 | 7.1 | 50.9 | 22.0 | 10.7 | 2.1 | 0.5 | 54.0 | 2.2 | 0.9 | 0.5 | 1.4 | 0.7 | 27.5 | ||
| 20 | 19.0 | 3.0 | 8.0 | 4.8 | 2.5 | 0.9 | 1.1 | 5.0 | 26.6 | 7.4 | 1.3 | 1.9 | 4.5 | 23.3 | 0.7 | 18.7 |
| 22 | 4.2 | 5.8 | 0.5 | 0.3 | 0.7 | 0.1 | 4.2 | 0.9 | 0.4 | 2.3 | 0.7 | 17.6 | ||||
| 24 | 2.3 | 17.1 | 1.1 | 8.8 | 1.8 | 1.0 | 5.5 | 4.4 | 81.3 | |||||||
| 26 | 4.0 | 5.6 | 0.9 | 3.1 | 1.1 | 0.5 | 0.9 | 5.5 | 41.2 | |||||||
| 28 | 4.1 | 3.1 | 0.1 | 0.9 | 0.2 | 3.4 | 20.2 | |||||||||
| 32 | 1.5 | 1.9 | 0.3 | 0.4 | 0.2 | 1.8 | 32.7 | |||||||||
| 34 | 1.0 | 1.1 | 0.1 | 0.5 | 0.7 | 0.3 | 0.2 | 62.9 | ||||||||
| 36 | 2.1 | 2.2 | 1.0 | 0.8 | 3.1 | 45.6 | ||||||||||
| PENDUELES | ||||||||||||||||
| 5 | 0.3 | 0.2 | 0.6 | 0.0 | 1.5 | |||||||||||
| 15 | 0.4 | 1.2 | 0.5 | 0.2 | 6.3 | |||||||||||
| 21 | 2.8 | 1.0 | 1.4 | 0.3 | 1.2 | 0.4 | ||||||||||
| 25 | 4.1 | 2.1 | 1.0 | |||||||||||||
| 29 | 3.1 | 5.4 | 3.1 | 1.7 | 5.2 | 1.4 | 0.2 | |||||||||
| 33 | 4.3 | 2.9 | 13.4 | 4.5 | 8.7 | 1.6 | 0.0 | 0.5 | ||||||||
| 37 | 6.2 | 3.3 | 2.7 | 1.8 | 0.1 | 1.8 | 0.3 | 0.5 | ||||||||
| 41 | 0.1 | 7.4 | 5.3 | 1.8 | 1.1 | 0.3 | 1.1 | 0.1 | 0.7 | |||||||
| 45 | 0.5 | 6.1 | 2.1 | 2.7 | 0.1 | 2.7 | 2.3 | |||||||||
| 49 | 14.9 | 1.7 | 7.1 | 5.0 | 31.6 | 8.3 | 0.6 | 0.3 | 5.5 | 0.2 | 11.0 | |||||
| 53 | 21.3 | 3.9 | 16.4 | 49.3 | 9.1 | 1.6 | 0.9 | 0.3 | 4.7 | 0.3 | 15.0 | |||||
| 57 | 13.2 | 3.1 | 21.6 | 11.9 | 23.5 | 6.4 | 1.5 | 0.3 | 0.4 | 9.6 | 0.5 | 26.4 | ||||
| 61 | 5.8 | 4.5 | 8.1 | 6.2 | 11.2 | 4.2 | 0.5 | 0.3 | 0.5 | 10.4 | 0.6 | 29.5 | ||||
| 65 | 2.3 | 2.9 | 11.5 | 3.9 | 1.2 | 3.2 | 0.5 | 0.2 | 10.4 | 0.2 | 7.3 | |||||
| 69 | 1.1 | 2.6 | 7.2 | 5.5 | 5.7 | 2.3 | 0.5 | 0.1 | 0.2 | 8.8 | 0.3 | 13.4 | ||||
| 73 | 1.8 | 2.3 | 10.7 | 4.6 | 6.8 | 2.2 | 0.6 | 0.3 | 0.2 | 9.8 | 0.1 | 4.8 | ||||
| 77 | 3.8 | 2.2 | 4.7 | 3.9 | 10.4 | 2.5 | 0.3 | 1.3 | 0.2 | 9.7 | 1.2 | |||||
| 81 | 11.2 | 1.8 | 4.1 | 2.4 | 2.5 | 0.7 | 0.7 | 5.4 | 0.3 | 12.2 | 2.3 | |||||
| 85 | 3.6 | 0.9 | 1.6 | 1.3 | 0.3 | 0.6 | 1.0 | 0.2 | 10.1 | 1.3 | ||||||
| 89 | 4.4 | 0.6 | 0.3 | 0.9 | 1.1 | 0.6 | 0.1 | 12.6 | 0.1 | 2.8 | ||||||
| 93 | 4.3 | 0.4 | 12.3 | 16.1 | 1.9 | 0.9 | 0.9 | 1.9 | 0.8 | 1.2 | 13.9 | 0.1 | 2.0 | |||
| 97 | 4.1 | 0.7 | 7.9 | 2.6 | 1.7 | 0.3 | 1.2 | 3.5 | 1.6 | 0.7 | 13.2 | 0.1 | 2.1 | |||
| 101 | 3.7 | 0.8 | 4.0 | 0.6 | 3.9 | 0.6 | 1.4 | 3.9 | 0.8 | 0.3 | 11.2 | 0.2 | 3.3 | |||
| 105 | 1.3 | 0.7 | 2.1 | 7.0 | 0.6 | 1.5 | 9.5 | 0.7 | 0.3 | 6.6 | 0.1 | 1.8 | ||||
| 109 | 0.4 | 0.3 | 0.2 | 0.6 | 0.2 | 0.8 | 4.3 | 0.3 | 0.9 | 4.1 | 0.3 | 3.9 | ||||
| 113 | 0.2 | 0.3 | 0.1 | 0.4 | 0.0 | 2.9 | 0.2 | 0.9 | 2.2 | 3.5 | ||||||
| 117 | 0.3 | 0.7 | 2.9 | 0.2 | 1.6 | 4.0 | 0.5 | 6.8 | ||||||||
| 121 | 0.3 | 0.5 | 0.5 | 0.3 | 0.7 | 4.6 | 0.8 | 1.7 | 3.4 | 0.5 | 6.3 | |||||
| 125 | 0.1 | 0.1 | 0.3 | 1.8 | 0.2 | 0.2 | 0.9 | 0.8 | 0.2 | 2.0 | ||||||
| 129 | 0.4 | 0.3 | 1.5 | 0.1 | 6.1 | 0.9 | 2.3 | 2.5 | ||||||||
| 133 | 0.3 | 0.3 | 0.2 | 0.4 | 0.9 | 0.3 | 0.3 | 0.9 | ||||||||
| Title | Roñanzas | Pendueles | Buelna | La Borbolla |
|---|---|---|---|---|
| n-Alkanes | −32.9 | −33.0 | −33.4 | −32.6 |
| σ n-Alkanes | 0.5 | 0.6 | 1.0 | 0.5 |
| n-Alkan-2-ones | −29.5 | −30.3 | −31.5 | −31.2 |
| σ n-Alkan-2-ones | 0.4 | 0.6 | 0.6 | 0.3 |
| Hopanoids | −25.9 | −27.2 | −27.9 | −27.5 |
| σ Hopanoids | 0.5 | 1.1 | 1.0 | 0.4 |
| Higher Plants Triterpenoids | −30.7 | −31.3 | -32.2 | −32.3 |
| σ Higher Plants Triterpenoids | 2.4 | 1.2 | 1.0 | 0.8 |
| Compound | R33 | R93 | R123 | R127 | R163 | R203 | R233 | PE25 | PE53 | PE77 | PE105 | PE121 | B216 | B228 | BU120 | BU132 | BU160 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olean-12-en-3β-ol acetate | −30.6 | ||||||||||||||||
| Urs-12-en-3β-ol acetate | −30.8 | ||||||||||||||||
| Lup-20(29)-en-3β-ol acetate | −30.4 | ||||||||||||||||
| Lupan-3-one | −29.8 | −30.0 | −30.3 | −29.8 | −29.6 | −30.6 | |||||||||||
| Friedelinol | −30.9 | ||||||||||||||||
| Friedelin | −32.1 | −31.8 | −31.5 | −30.9 | −31.9 | ||||||||||||
| Friedelinol acetate | −29.5 | −30.1 | |||||||||||||||
| Bauer-7-en-3-one | −31.4 | ||||||||||||||||
| Bauer-7-en-3β-ol acetate | −32.3 | −32.2 | −33.1 | −32.6 | −33.3 | ||||||||||||
| Bauerenol acetate isomer | −32.9 | −31.5 | −32.5 | ||||||||||||||
| β C27 Hopane | −28.1 | −27.9 | −28.0 | −27.6 | −26.3 | ||||||||||||
| αβ C31 Hopane | −25.9 | −25.4 | −24.9 | −26.6 | −26.2 | −25.7 | −26.0 | −25.8 | −27.9 | −28.4 | −26.3 | −27.2 | −27.4 | −28.3 | |||
| ββ C31 Hopane | −26.3 | −26.0 | −25.3 | −26.3 | |||||||||||||
| 22,29,30-Trisnorhopan-21-one | −27.8 | −27.1 | |||||||||||||||
| Hop-22(29)-ene | −26.1 | ||||||||||||||||
| Hop-22(29)-en-3-one | −26.4 | −26.2 | −27.7 | −27.4 | |||||||||||||
| Hop-17(21)-en-3β-ol acetate | −26.5 | −27.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Días, V.; Borrego, A.G.; Blanco, C.G.; Bechtel, A.; Püttmann, W. Significance of the High Abundance of Pentacyclic Triterpenyl and Hopenyl Acetates in Sphagnum Peat Bogs from Northern Spain. Quaternary 2019, 2, 30. https://0-doi-org.brum.beds.ac.uk/10.3390/quat2030030
López-Días V, Borrego AG, Blanco CG, Bechtel A, Püttmann W. Significance of the High Abundance of Pentacyclic Triterpenyl and Hopenyl Acetates in Sphagnum Peat Bogs from Northern Spain. Quaternary. 2019; 2(3):30. https://0-doi-org.brum.beds.ac.uk/10.3390/quat2030030
Chicago/Turabian StyleLópez-Días, Veneranda, Angeles G. Borrego, Carlos G. Blanco, Achim Bechtel, and Wilhelm Püttmann. 2019. "Significance of the High Abundance of Pentacyclic Triterpenyl and Hopenyl Acetates in Sphagnum Peat Bogs from Northern Spain" Quaternary 2, no. 3: 30. https://0-doi-org.brum.beds.ac.uk/10.3390/quat2030030