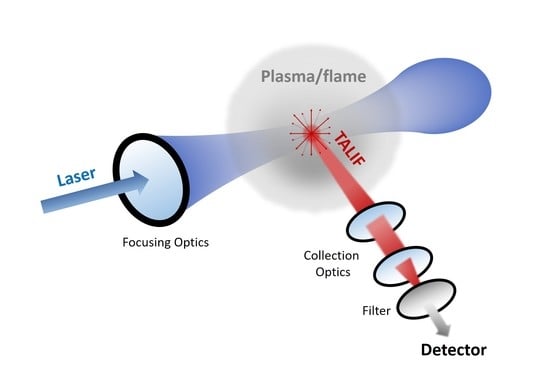
Progresses on the Use of Two-Photon Absorption Laser Induced Fluorescence (TALIF) Diagnostics for Measuring Absolute Atomic Densities in Plasmas and Flames
Abstract
:1. Introduction
2. LIF and TALIF Spectroscopy
2.1. LIF Concept
2.2. TALIF Principles, Involved Theory and Experimental Requirements
| Conditions | Pulse Duration/Energy | Pulse Frequency | Species: Fluorescence Transition | Laser/TALIF Wavelength (nm) | Reference |
|---|---|---|---|---|---|
| Laser Used | |||||
| ArF | 4–5 ns/2 mJ | 10 Hz | H: n = 3 n = 2 | 205/656.3 | [33] |
| Nd:YAG + Dye | 10 ns/0.5 mJ | 20 Hz | H: n = 3 n = 2 | 205/656.3 | [63] |
| Nd:YAG + Dye | -/50–60 μJ | - | H: 3d2D3/2,5/2 2p2P1/2,3/2 | 205.08/656.3 | [64] |
| Nd:YAG + Dye | 6 ns/0.3–0.7 mJ | 10 Hz | H: 3d2D3/2,5/2 2p2P1/2,3/2 | 205/656.3 | [65] |
| Nd:YAG + Dye | 6 ns/250 μJ | 10 Hz | H: 3d2D3/2,5/2 2p2P1/2,3/2 | 205.08/656 | [42] |
| Nd:YAG + Dye | -/4 mJ | - | N: 2p23p4D02p23s4P | 211/869 | [48] |
| Nd:YAG + Dye | -/150 μJ | - | N: (3p)4(3s)4P | 207/745 | [66] |
| Nd:YAG + Dye | 5 ns/130 μJ | 20 Hz | N: (3p)4(3s)4P1/2,3/2,5/2 | 206.7/742–747 | [67] |
| Nd:YAG + Dye | 5 ns/1 mJ | 10 Hz | N: (3p)4(3s)4P1/2,3/2,5/2 | 206.65/742–746 | [68] |
| Nd:YAG + Dye | -/80–370 μJ | - | N: (3s)4(3s)4P5/2 N: (3p)4(3s)4P | 211/868 207/745 | [69] |
| Nd:YAG + Dye | 10 ns/5 mJ | 10 Hz | N: (3p)4(3s)4P1/2,3/2,5/2 | 206.72/742–746 | [70] |
| Nd:YAG + Dye | 8 ns/- | - | N: (3p)4(3s)4P1/2,3/2,5/2 | 206.65/744 | [71] |
| Nd:YAG + Dye | 6.5 ns/3 mJ | 10 Hz | N: (3p)4(3s)4P1/2,3/2,5/2 | 206.7/742–746 | [72] |
| Nd:YAG + Dye | 5 ns/2.5 mJ | - | O: 3p3P3s3S | 226/845 | [73] |
| Excimer + Dye | -/150 μJ | - | O: 3p5P3s5S | 226/777 | [74] |
| Nd:YAG + Dye | 5 ns/5.2 mJ | - | O: 3p5P3s5S O: 3p3P3s3S | 226/777 226/845 | [75] |
| Nd:YAG + Dye | -/185 μJ | - | O: 3p3P3s3S | 226/845 | [76] |
| Nd:YAG + Dye | -/350 μJ | - | O: 3p3P23s3S1 | 225.582/844.6 | [77] |
| Nd:YAG + Dye | 5 ns/4 mJ | 10 Hz | O: 3p3P1,2,03s3S | 225.65/844.87 | [78] |
| Nd:YAG + Dye | 5 ns/4 mJ | 10 Hz | O: 3p3P1,2,03s3S | 225/844.87 | [79] |
| Nd:YAG + Dye | 5 ns/4 mJ | 10 Hz | O: 3p3P1,2,03s3S | 225.65/844.87 | [80] |
| Nd:YAG + Dye | -/1–3 μJ | 1–2 kHz | O: 3p3P1,2,03s3S | 225.586/844.68 | [61] |
| Nd:YAG + Dye | -/>0.1 mJ | - | O: 3p3P1,2,03s3S | 226/844.68 | [81] |
| Nd:YAG + Dye | -/1–200 μJ | 10 Hz | O: 3p3P1,2,03s3S1 | 225.58/844.6 | [82] |
| Tuneable diode Nd:YAG + Dye | -/- -/250 μJ | 30 MHz 20 Hz | O: 3p3P1,2,03s3S1 | 225.6/844.6 | [83] |
| Nd:YAG + Dye | 5 ns/0.3–0.5 mJ | 10 Hz | O: 3p3P1,2,03s3S1 | 225.58/844.6 | [84] |
| Nd:YAG + Dye | 20 ns/<0.4 mJ | - | O: 3p3P3s3S | 225.6/844.6 | [85] |
| Nd:YAG | 7 ns/50–100 μJ | - | O: 3p3P3s3S N: 2p23p4D02p23s4P | 226/845 211/869 | [35] |
| Nd:YAG + Dye | 7 ns/3 mJ | - | O: 3p3P3s3S Hα: n = 3 n = 2 | 226/845 205/656.3 | [86] |
| Nd:YAG + Dye | 8 ns/0.5 mJ | 10 Hz | H: 3d2D3/2,5/2 2p2P1/2,3/2 N: (3p)4(3s)4P1/2,3/2,5/2 O: 3p3P1,2,03s3S | 205.08/656.3 206.65/742–746 225.58/844.64 | [36] |
| Nd:YAG + Dye | -/200 μJ | - | N: (3s)4(3s)4P5/2 N: (3p)4(3s)4P O: 3p3P23s3S1 | 210.788/868.027 206.718/746.83 225.582/844.6 | [87] |
| Conditions | Pulse Duration/Energy | Pulse Frequency | Species: Fluorescence Transition | Laser/TALIF Wavelength (nm) | Reference |
|---|---|---|---|---|---|
| Laser Used | |||||
| Nd:YAG+ Dye | 10 ps/20 μJ | 10 Hz | H: n = 3 n = 2 | 205/656.3 | [48] |
| Nd:YAG+ Dye | 70 ps/0.3 mJ | 20 Hz | H: n = 3 n = 2 | 205/656.3 | [88] |
| Nd:YAG+ Dye | 100 ps/0.4 mJ | 20 Hz | H: n = 3 n = 2 | 205.144/656.3 | [53] |
| Nd:YLF Ti: Sapphire | 100 fs/62 mW | 1 kHz | N: (3p)4(3s)4P1/2,3/2,5/2 | 206.65/742–746 | [62] |
| Nd:YAG+ Dye | 10 ps/40 20 μJ | 10 Hz | O: 3p3P3s3S | 226/845 | [47] |
| Nd:YAG+ Dye | 55 ps/3 μJ | 20 Hz | O: 3p3P3s3S | 225.655/845 | [89] |
| Nd:YAG+ Dye | 55 ps/2–1200 μJ | 20 Hz | O: 3p3P3s3S | 225.655/845 | [52] |
| Nd:YAG+ Dye | 100 ps/600 μJ | - | O: 3p3P3s3S | 225.655/845 | [51] |
| Ti: Sapphire | 100 fs/13 μJ | 1 kHz | O: 3p3P1,2,03s3S | 225.65/844.87 | [45] |
| Ti: Sapphire | 100 fs/12 μJ | 1 kHz | O: 3p3P1,2,03s3S | 225.59/844.65 | [90] |
| Nd:YAG | 30 ps/35 μJ 30ps/24 μJ | 10 Hz | Hα: n = 3 n = 2 O: 3p3P1,2,03s3S1 | 205.08/656.3 225.65/844.6 | [44] |
| Ti: Sapphire | 100 fs/10 μJ 100 fs/15 μJ | 10 kHz 1 kHz | Hα: n = 3 n = 2 O: 3p3P1,2,03s3S | 205.08/656.27 225.59/844.65 | [91] |
- cm4, which is the atomic lineshape-independent cross-section () depending only on the nature of the atom;
- cm4·s, resulting from the product: G(2);
- cm4·W−1, resulting from the product: 2g()G(2)/hν, ν being the laser photon frequency.
- The depletion by the laser radiation of the species ground-state by two-photon absorption is negligible. This is true when the corresponding inverse of the two-photon absorption rate (i.e., 1/, units in sec) is much higher than the duration of the laser pulse.
- The depletion by photoionization (PIN) and amplified stimulated emission (ASE) of the laser-excited state is significantly lower than that due to spontaneous emission (A) and collisional quenching (Q). The implication of those processes in the TALIF scheme will be discussed in more detail in the next section.
3. Fast (ns) and Ultrafast (ps/fs) TALIF: Probing Atomic Species in Plasmas, Flames, and/or Gases
3.1. Fast (ns) TALIF
3.2. Ultrafast (ps/fs) TALIF
4. Challenges on the Use of Fast (ns) and Ultrafast (ps/fs) TALIF for Diagnostic Purposes
4.1. Challenges When Using ns–TALIF in Flames and Gases
4.2. Challenges When Using ps/fs–TALIF in Flames and Gases
4.3. Challenges When Using ns– and ps/fs–TALIF in Plasmas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertolotti, M. The History of the Laser; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Gamaly, E.G.; Rode, A.V. Physics of ultra-short laser interaction with matter: From phonon excitation to ultimate transformations. Prog. Quantum Electron. 2013, 37, 215–323. [Google Scholar] [CrossRef]
- Demtröder, W. Laser Spectroscopy Vol. 1: Basic Principles; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Wetzig, A.; Herwig, P.; Hauptmann, J.; Baumann, R.; Rauscher, P.; Schlosser, M.; Pinder, T.; Leyens, C. Fast laser cutting of thin metal. Procedia Manuf. 2019, 29, 369–374. [Google Scholar] [CrossRef]
- Bachmann, M.; Gumenyuk, A.; Rethmeier, M. Welding with high-power lasers: Trends and developments. Phys. Procedia 2016, 83, 15–25. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Atzeni, S.; Pentangelo, C.; Pellegatta, F.; Crespi, A.; Osellame, R. Low power reconfigurability and reduced crosstalk in integrated photonic circuits fabricated by femtosecond laser micromachining. Laser Photonics Rev. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Luke, A.M.; Mathew, S.; Altawash, M.M.; Madan, B.M. Lasers: A review with their applications in oral medicine. J. Lasers Med. Sci. 2019, 10, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalkhal, E.; Rezaei-Tavirani, M.; Zali, M.R.; Akbari, Z. The evaluation of laser application in surgery: A review article. J. Lasers Med. Sci. 2019, 10, S104–S111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jipa, F.; Zamfirescu, M.; Velea, A.; Popescu, M.; Dabu, R. Updates in Advanced Lithography (Chapter 3: Femtosecond Laser Lithography in Organic and Non-Organic Materials); IntechOpen: Rijeka, Croatia, 2013; ISBN 978-953-51-5709-0. [Google Scholar] [CrossRef] [Green Version]
- Gazeli, O.; Bellou, E.; Stefas, D.; Couris, S. Laser-based classification of olive oils assisted by machine learning. Food Chem. 2020, 302, 125329. [Google Scholar] [CrossRef]
- Lefort, C. A review of biomedical multiphoton microscopy and its laser sources. J. Phys. D Appl. Phys. 2017, 50, 423001. [Google Scholar] [CrossRef] [Green Version]
- Hübner, S.; Sousa, J.S.; van der Mullen, J.; Graham, W.G. Thomson scattering on non-thermal atmospheric pressure plasma jets. Plasma Sources Sci. Technol. 2015, 24, 054005. [Google Scholar] [CrossRef] [Green Version]
- Verreycken, T.; van Gessel, A.F.H.; Pageau, A.; Bruggeman, P. Validation of gas temperature measurements by OES in an atmospheric air glow discharge with water electrode using Rayleigh scattering. Plasma Sources Sci. Technol. 2011, 20, 024002. [Google Scholar] [CrossRef] [Green Version]
- Gazeli, K.; Bauville, G.; Fleury, M.; Jeanney, P.; Neveu, O.; Pasquiers, S.; Santos Sousa, J. Effect of the gas flow rate on the spatiotemporal distribution of Ar(1s5) absolute densities in a ns pulsed plasma jet impinging on a glass surface. Plasma Sources Sci. Technol. 2018, 27, 065003. [Google Scholar] [CrossRef]
- Gazeli, K.; Vazquez, T.; Al-Homsi, S.; Bauville, G.; Blin-Simiand, N.; Bournonville, B.; Fleury, M.; Jeanney, P.; Neveu, O.; Pasquiers, S.; et al. Ar(1s5) absolute radial densities in a ns-pulsed argon plasma jet impinging on dielectric targets at floating potential–plasma action on organic molecules. Plasma Process. Polym. 2018, 15, e1800080. [Google Scholar] [CrossRef]
- Gazeli, K.; Vazquez, T.; Bauville, G.; Blin-Simiand, N.; Bournonville, B.; Pasquiers, S.; Santos Sousa, J. Experimental investigation of a ns-pulsed argon plasma jet for the fast desorption of weakly volatile organic compounds deposited on glass substrates at variable electric potential. J. Phys. D Appl. Phys. 2020, 53, 475202. [Google Scholar] [CrossRef]
- Darny, T.; Pouvesle, J.; Puech, V.; Douat, C.; Dozias, S.; Robert, E. Analysis of conductive target influence in plasma jet experiments through helium metastable and electric field measurements. Plasma Sources Sci. Technol. 2017, 26, 045008. [Google Scholar] [CrossRef]
- Chng, T.L.; Orel, I.; Adamovich, I.V.; Popov, N.A.; Starikovskaia, S. N-atom production at high electric fields: E-FISH and TALIF experiments for understanding fast ionization wave kinetics. AIAA Scitech Forum 2020. [Google Scholar] [CrossRef]
- Chng, T.L.; Brisset, A.; Jeanney, P.; Starikovskaia, S.M.; Adamovich, I.V.; Tardiveau, P. Electric field evolution in a diffuse ionization wave nanosecond pulse discharge in atmospheric pressure air. Plasma Sources Sci. Technol. 2019, 28, 09LT02. [Google Scholar] [CrossRef]
- Goldberg, B.M.; Reuter, S.; Dogariu, A.; Miles, R.B. 1-D spatially resolved electric fields in atmospheric pressure nanosecond pulse discharges using ultrashort laser pulses. AIAA Scitech Forum 2019. [Google Scholar] [CrossRef]
- Dogariu, L.E.; Dogariu, A.; Miles, R.B.; Smith, M.S.; Marineau, E.C. Femtosecond laser electronic excitation tagging velocimetry in a large-scale hypersonic facility. AIAAJ 2019, 57, 4725–4737. [Google Scholar] [CrossRef]
- Moreau, E.; Audier, P.; Orriere, T.; Benard, N. Electrohydrodynamic gas flow in a positive corona discharge. J. Appl. Phys. 2019, 125, 133303. [Google Scholar] [CrossRef]
- Stancu, G.D.; Kaddouri, F.; Lacoste, D.A.; Laux, C.O. Atmospheric pressure plasma diagnostics by OES, CRDS and TALIF. J. Phys. D Appl. Phys. 2010, 43, 124002. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Adamovich, I.; Gord, J.R.; Roy, S. Recent advances in ultrafast-laser-based spectroscopy and imaging for reacting plasmas and flames. Plasma Sources Sci. Technol. 2017, 26, 103001. [Google Scholar] [CrossRef]
- Dilecce, G.; Martini, L.M.; Tosi, P.; Scotoni, M.; De Benedictis, S. Laser induced fluorescence in atmospheric pressure discharges. Plasma Sources Sci. Technol. 2015, 24, 034007. [Google Scholar] [CrossRef] [Green Version]
- Tango, W.J.; Link, J.K.; Zare, R.N. Spectroscopy of K2 using laser-induced fluorescence. J. Chem. Phys. 1968, 49, 4264–4268. [Google Scholar] [CrossRef]
- Bruggeman, P.; Brandenburg, R. Atmospheric pressure discharge filaments and microplasmas: Physics, chemistry and diagnostics. J. Phys. D Appl. Phys. 2013, 46, 464001. [Google Scholar] [CrossRef]
- Amorim, J.; Baravian, G.; Jolly, J. Laser-induced resonance fluorescence as a diagnostic technique in non-thermal equilibrium plasmas. J. Phys. D Appl. Phys. 2000, 33, R51–R65. [Google Scholar] [CrossRef]
- Döbele, H.F.; Mosbach, T.; Niemi, K.; Schulz-von der Gathen, V. Laser-induced fluorescence measurements of absolute atomic densities: Concepts and limitations. Plasma Sources Sci. Technol. 2005, 14, S31–S41. [Google Scholar] [CrossRef] [Green Version]
- Telle, H.H.; Urena, A.G.; Donovan, R.J. Laser Chemistry: Spectroscopy, Dynamics and Applications; John Wiley & Sons Ltd.: West Sussex, UK, 2007; ISBN 978-0-471-48570-4 (HB). [Google Scholar]
- Herzberg, G. Molecular Spectra and Molecular Structure I; Spectra of Diatomic Molecules; D. Van Nostrand Company, INC.: Princeton, NJ, USA, 1950. [Google Scholar]
- Ouaras, K.; Magne, L.; Pasquiers, S.; Tardiveau, P.; Jeanney, P.; Bournonville, B. OH density measured by PLIF in a nanosecond atmospheric pressure diffuse discharge in humid air under steep high voltage pulses. Plasma Sources Sci. Technol. 2018, 27, 045002. [Google Scholar] [CrossRef]
- Bokor, J.; Freeman, R.R.; White, J.C.; Storz, R.H. Two-photon excitation of the n=3 level in H and D atoms. Phys. Rev. A 1981, 24, 612–614. [Google Scholar] [CrossRef]
- Bischel, W.K.; Perry, B.E.; Crosley, D.R. Two-photon laser-induced fluorescence in oxygen and nitrogen atoms. Chem. Phys. Lett. 1981, 82, 85–88. [Google Scholar] [CrossRef]
- Bischel, W.K.; Perry, B.E.; Crosley, D.R. Detection of fluorescence from O and N atoms induced by two-photon absorption. Appl. Opt. 1982, 21, 1419. [Google Scholar] [CrossRef] [PubMed]
- Niemi, K.; Schulz-von der Gathen, V.; Döbele, H.F. Absolute calibration of atomic density measurements by laser-induced fluorescence spectroscopy with two-photon excitation. J. Phys. D Appl. Phys. 2001, 34, 2330–2335. [Google Scholar] [CrossRef] [Green Version]
- Aldén, M.; Edner, H.; Grafström, P.; Svanberg, S. Two-photon excitation of atomic oxygen in a flame. Opt. Commun. 1982, 42, 244–246. [Google Scholar] [CrossRef]
- Goldsmith, J.E.M. Two-photon-excited stimulated emission from atomic hydrogen in flames. J. Opt. Soc. Am. B 1989, 6, 1979. [Google Scholar] [CrossRef]
- Agrup, S.; Westblom, U.; Aldén, M. Detection of atomic nitrogen using two-photon laser-induced stimulated emission: Application to flames. Chem. Phys. Lett. 1990, 170, 406–410. [Google Scholar] [CrossRef]
- Es-sebbar, E.T.; C-Gazeau, M.; Benilan, Y.; Jolly, A.; Pintassilgo, C.D. Absolute ground-state nitrogen atom density in a N2/CH4 late afterglow: TALIF experiments and modelling studies. J. Phys. D Appl. Phys. 2010, 43, 335203. [Google Scholar] [CrossRef]
- Duan, X.R.; Lange, H.; Meyer-Plath, A. Absolute density distribution of H atoms in a large-scale microwave plasma reactor. Plasma Sources Sci. Technol. 2003, 12, 554–560. [Google Scholar] [CrossRef]
- Yue, Y.; Kondeti, V.S.S.K.; Bruggeman, P.J. Absolute atomic hydrogen density measurements in an atmospheric pressure plasma jet: Generation, transport and recombination from the active discharge region to the effluent. Plasma Sources Sci. Technol. 2020, 29, 04LT01. [Google Scholar] [CrossRef]
- Dumitrache, C.; Gallant, A.; Stancu, G.D.; Laux, C.O. Ground-state atomic nitrogen measurements using fs-TALIF in high-pressure NRP discharges. AIAA Scitech Forum 2020. [Google Scholar] [CrossRef]
- Schröter, S.; Bredin, J.; Gibson, A.R.; West, A.; Dedrick, J.P.; Wagenaars, E.; Niemi, K.; Gans, T.; O’Connell, D. The formation of atomic oxygen and hydrogen in atmospheric pressure plasmas containing humidity: Picosecond two-photon absorption laser induced fluorescence and numerical simulations. Plasma Sources Sci. Technol. 2020, 29, 105001. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Sands, B.L.; Kulatilaka, W.D.; Roy, S.; Scofield, J.; Gord, J.R. Femtosecond, two-photon laser-induced-fluorescence imaging of atomic oxygen in an atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 2015, 24, 032004. [Google Scholar] [CrossRef]
- Westblom, U.; Agrup, S.; Aldén, M.; Cederbalk, P. Detection of nitrogen atoms in flames using two-photon laser-induced fluorescence and investigations of photochemical effects. Appl. Opt. 1991, 30, 2990. [Google Scholar] [CrossRef] [PubMed]
- Agrup, S.; Aldén, M. Two-photon laser-induced fluorescence and stimulated emission measurements from oxygen atoms in a hydrogen/oxygen flame with picosecond resolution. Opt. Commun. 1994, 113, 315–323. [Google Scholar] [CrossRef]
- Agrup, S.; Ossler, F.; Aldén, M. Measurements of collisional quenching of hydrogen atoms in an atmospheric-pressure hydrogen oxygen flame by picosecond laser-induced fluorescence. Appl. Phys. B Lasers Opt. 1995, 61, 479–487. [Google Scholar] [CrossRef]
- Amorim, J.; Baravian, G.; Touzeau, M.; Jolly, J. Two-photon laser induced fluorescence and amplified spontaneous emission atom concentration measurements in O2 and H2 discharges. J. Appl. Phys. 1994, 76, 1487–1493. [Google Scholar] [CrossRef]
- Lukas, C.; Spaan, M.; Der Gathen, V.S.-V.; Thomson, M.; Wegst, R.; Döbele, H.F.; Neiger, M. Dielectric barrier discharges with steep voltage rise: Mapping of atomic nitrogen in single filaments measured by laser-induced fluorescence spectroscopy. Plasma Sources Sci. Technol. 2001, 10, 445–450. [Google Scholar] [CrossRef]
- Frank, J.H.; Settersten, T.B. Two-photon LIF imaging of atomic oxygen in flames with picosecond excitation. Proc. Combust. Inst. 2005, 30, 1527–1534. [Google Scholar] [CrossRef]
- Frank, J.H.; Chen, X.; Patterson, B.D.; Settersten, T.B. Comparison of nanosecond and picosecond excitation for interference-free two-photon laser-induced fluorescence imaging of atomic oxygen in flames. Appl. Opt. 2004, 43, 2588. [Google Scholar] [CrossRef]
- Kulatilaka, W.D.; Frank, J.H.; Settersten, T.B. Interference-free two-photon LIF imaging of atomic hydrogen in flames using picosecond excitation. Proc. Combust. Inst. 2009, 32, 955–962. [Google Scholar] [CrossRef]
- Kulatilaka, W.D.; Gord, J.R.; Katta, V.R.; Roy, S. Photolytic-interference-free, femtosecond two-photon fluorescence imaging of atomic hydrogen. Opt. Lett. 2012, 37, 3051. [Google Scholar] [CrossRef]
- Stancu, G.D. Two-photon absorption laser induced fluorescence: Rate and density-matrix regimes for plasma diagnostics. Plasma Sources Sci. Technol. 2020, 29, 054001. [Google Scholar] [CrossRef]
- Loudon, R. The Quantum Theory of Light; Clarendon: Oxford, UK, 1983. [Google Scholar]
- Masalov, A.V. Spectral and temporal fluctuations of broadband laser radiation. Prog. Opt. 1985, 22, 145–196. [Google Scholar]
- Payne, M.G.; Chen, C.H.; Hurst, G.S.; Foltz, G.W. Applications of resonance ionization spectroscopy in atomic and molecular physics. Adv. At. Mol. Opt. 1982, 17, 229–274. [Google Scholar]
- Bamford, D.J.; Jusinski, L.E.; Bischel, W.K. Absolute two-photon absorption and three-photon ionization cross sections for atomic oxygen. Phys. Rev. A 1986, 34, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Kroll, S.; Aldén, M.; Bengtsson, P.-E.; Lofström, C.; Hall, R.J. Statistics of multimode YAG laser radiation with implications for quantitative coherent anti-Stokes Raman scattering spectroscopy in combustion diagnostics. J. Opt. Soc. Am. B 1991, 8, 5. [Google Scholar] [CrossRef]
- Van Gessel, A.F.H.; Van Grootel, S.C.; Bruggeman, P.J. Atomic oxygen TALIF measurements in an atmospheric-pressure microwave plasma jet with in situ xenon calibration. Plasma Sources Sci. Technol. 2013, 22, 055010. [Google Scholar] [CrossRef]
- Dumitrache, C.; Gallant, A.; Stancu, G.D.; Laux, C.O. Femtosecond two-photon absorption laser induced fluorescence (fs-TALIF) imaging of atomic nitrogen in nanosecond repetitive discharges. AIAA Scitech Forum 2019. [Google Scholar] [CrossRef]
- Tserepi, A.D.; Dunlop, J.R.; Preppernau, B.L.; Miller, T.A. Absolute H-atom concentration profiles in continuous and pulsed rf discharges. J. Appl. Phys. 1992, 72, 2638–2643. [Google Scholar] [CrossRef]
- Mrkvičková, M.; Ráheľ, J.; Dvořák, P.; Trunec, D.; Morávek, T. Fluorescence (TALIF) measurement of atomic hydrogen concentration in a coplanar surface dielectric barrier discharge. Plasma Sources Sci. Technol. 2016, 25, 055015. [Google Scholar] [CrossRef] [Green Version]
- Yatom, S.; Luo, Y.; Xiong, Q.; Bruggeman, P.J. Nanosecond pulsed humid Ar plasma jet in air: Shielding, discharge characteristics and atomic hydrogen production. J. Phys. D Appl. Phys. 2017, 50, 415204. [Google Scholar] [CrossRef]
- Adams, S.F.; Miller, T.A. Two-photon absorption laser-induced fluorescence of atomic nitrogen by an alternative excitation scheme. Chem. Phys. Lett. 1998, 295, 305–311. [Google Scholar] [CrossRef]
- Mazouffre, S.; Bakker, I.; Vankan, P.; Engeln, R.; Schram, D.C. Two-photon laser induced fluorescence spectroscopy performed on free nitrogen plasma jets. Plasma Sources Sci. Technol. 2002, 11, 439–447. [Google Scholar] [CrossRef]
- Wagenaars, E.; Gans, T.; O’Connell, D.; Niemi, K. Two-photon absorption laser-induced fluorescence measurements of atomic nitrogen in a radio-frequency atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 2012, 21, 042002. [Google Scholar] [CrossRef]
- Ono, R.; Teramoto, Y.; Oda, T. Measurement of atomic nitrogen in N2 pulsed positive corona discharge using two-photon absorption laser-induced fluorescence. Jpn. J. Appl. Phys. 2009, 48, 122302. [Google Scholar] [CrossRef]
- Es-Sebbar, E.T.; Gherardi, N.; Massines, F. Effects of N2O and O2 addition to nitrogen Townsend dielectric barrier discharges at atmospheric pressure on the absolute ground-state atomic nitrogen density. J. Phys. D Appl. Phys. 2013, 46, 015202. [Google Scholar] [CrossRef]
- Dvořák, P.; Šimek, M.; Prukner, V. Evolution of N(4S) atoms produced under nitrogen streamer conditions: Time-resolved TALIF study at reduced pressures. Plasma Sources Sci. Technol. 2019, 28, 125004. [Google Scholar] [CrossRef]
- Chng, T.L.; Lepikhin, N.D.; Orel, I.S.; Popov, N.A.; Starikovskaia, S.M. TALIF measurements of atomic nitrogen in the afterglow of a nanosecond capillary discharge. Plasma Sources Sci. Technol. 2020, 29, 035017. [Google Scholar] [CrossRef]
- Goldsmith, J.E.M. Photochemical effects in two-photon-excited fluorescence detection of atomic oxygen in flames. Appl. Opt. 1987, 26, 3566. [Google Scholar] [CrossRef] [PubMed]
- Wysong, I.J.; Jeffries, J.B.; Crosley, D.R. Laser-induced fluorescence of O(3p3P), O2, and NO near 226 nm: Photolytic interferences and simultaneous excitation in flames. Opt. Lett. 1989, 14, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Van Oostendorp, D.L.; Levinsky, H.B.; van der Meij, C.E.; Jacobs, R.A.A.M.; Borghols, W.T.A. Avoidance of the photochemical production of oxygen atoms in one-dimensional, two-photon laser-induced fluorescence imaging. Appl. Opt. 1993, 32, 4636. [Google Scholar] [CrossRef] [PubMed]
- Tserepi, A.D.; Wurzberg, E.; Miller, T.A. Two-photon-excited stimulated emission from atomic oxygen in rf plasmas: Detection and estimation of its threshold. Chem. Phys. Lett. 1997, 265, 297–302. [Google Scholar] [CrossRef]
- Dilecce, G.; Vigliotti, M.; De Benedictis, S. A TALIF calibration method for quantitative oxygen atom density measurement in plasma jets. J. Phys. D Appl. Phys. 2000, 33, L53–L56. [Google Scholar] [CrossRef]
- Niemi, K.; Schulz-von der Gathen, V.; Döbele, H.F. Absolute atomic oxygen density measurements by two-photon absorption laser-induced fluorescence spectroscopy in an RF-excited atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2005, 14, 375–386. [Google Scholar] [CrossRef]
- Waskoenig, J.; Niemi, K.; Knake, N.; Graham, L.M.; Reuter, S.; Schulz-von der Gathen, V.; Gans, T. Atomic oxygen formation in a radio-frequency driven micro-atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2010, 19, 045018. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; Winter, J.; Schmidt-Bleker, A.; Schroeder, D.; Lange, H.; Knake, N.; Schulz-von der Gathen, V.; Weltmann, K.-D. Atomic oxygen in a cold argon plasma jet: TALIF spectroscopy in ambient air with modelling and measurements of ambient species diffusion. Plasma Sources Sci. Technol. 2012, 21, 024005. [Google Scholar] [CrossRef]
- Jia, F.; Ishikawa, K.; Takeda, K.; Kano, H.; Kularatne, J.; Kondo, H.; Sekine, M.; Hori, M. Spatiotemporal behaviors of absolute density of atomic oxygen in a planar type of Ar/O2 non-equilibrium atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 2014, 23, 025004. [Google Scholar] [CrossRef]
- Klochko, A.V.; Lemainque, J.; Booth, J.P.; Starikovskaia, S.M. TALIF measurements of oxygen atom density in the afterglow of a capillary nanosecond discharge. Plasma Sources Sci. Technol. 2015, 24, 025010. [Google Scholar] [CrossRef] [Green Version]
- Marinov, D.; Drag, C.; Blondel, C.; Guaitella, O.; Golda, J.; Klarenaar, B.; Engeln, R.; Schulz-von der Gathen, V.; Booth, J.P. Pressure broadening of atomic oxygen two-photon absorption laser induced fluorescence. Plasma Sources Sci. Technol. 2016, 25, 06LT03. [Google Scholar] [CrossRef]
- Li, D.; Kong, M.G.; Britun, N.; Snyders, R.; Leys, C.; Nikiforov, A. Quantitative measurements of ground state atomic oxygen in atmospheric pressure surface micro-discharge array. J. Phys. D Appl. Phys. 2017, 50, 215201. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kawakita, T.; Uchida, S.; Tochikubo, F. Simultaneous measurement of local densities of atomic oxygen and ozone in pure oxygen pulsed barrier discharge under atmospheric pressure. J. Phys. D Appl. Phys. 2020, 53, 135201. [Google Scholar] [CrossRef]
- Meler, U.; Kohse-Höinghaus, K.; Just, T. H and O atom detection for combustion applications: Study of quenching and laser photolysis effects. Chem. Phys. Lett. 1986, 126, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Broc, A.; De Benedictis, S.; Dilecce, G. LIF investigations on NO, O and N in a supersonic N2/O2/NO RF plasma jet. Plasma Sources Sci. Technol. 2004, 13, 504–514. [Google Scholar] [CrossRef]
- Kulatilaka, W.D.; Patterson, B.D.; Frank, J.H.; Settersten, T.B. Comparison of nanosecond and picosecond excitation for interference-free two-photon laser-induced fluorescence detection of atomic hydrogen in flames. Appl. Opt. 2008, 47, 4672. [Google Scholar] [CrossRef] [PubMed]
- Settersten, T.B.; Dreizler, A.; Patterson, B.D.; Schrader, P.E.; Farrow, R.L. Photolytic interference affecting two-photon laser-induced fluorescence detection of atomic oxygen in hydrocarbon flames. Appl. Phys. B Lasers Opt. 2003, 76, 479–482. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Sands, B.; Scofield, J.; Gord, J.R.; Roy, S. Comparison of femtosecond- and nanosecond-two-photon-absorption laser-induced fluorescence (TALIF) of atomic oxygen in atmospheric-pressure plasmas. Plasma Sources Sci. Technol. 2017, 26, 055004. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Roy, S.; Kulatilaka, W.D.; Shkurenkov, I.; Adamovich, I.V.; Lempert, W.R.; Gord, J.R. Femtosecond, two-photon-absorption, laser-induced-fluorescence (fs-TALIF) imaging of atomic hydrogen and oxygen in non-equilibrium plasmas. J. Phys. D Appl. Phys. 2017, 50, 015204. [Google Scholar] [CrossRef] [Green Version]
- Goehlich, A.; Kawetzki, T.; Döbele, H.F. On absolute calibration with xenon of laser diagnostic methods based on two-photon absorption. J. Chem. Phys. 1998, 108, 9362–9370. [Google Scholar] [CrossRef]
- Saxon, R.P.; Eichler, J. Theoretical calculation of two-photon absorption cross sections in atomic oxygen. Phys. Rev. A 1986, 34, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gontier, Y.; Trahin, M. On the multiphoton absorption in atomic hydrogen. Phys. Lett. A 1971, 36, 463–464. [Google Scholar] [CrossRef]
- Tung, J.H.; Tang, A.Z.; Salamo, G.J.; Chan, F.T. Two-photon absorption of atomic hydrogen from two light beams. J. Opt. Soc. Am. B 1986, 3, 837. [Google Scholar] [CrossRef]
- Gontier, Y.; Trahin, M. Multiphoton processes in a hydrogen atom. Phys. Rev. A 1971, 4, 1896–1906. [Google Scholar] [CrossRef]
- Gazeli, K.; Aubert, X.; Swaminathan, P.; Duluard, C.Y.; Lombardi, G.; Hassouni, K. Picosecond two-photon laser induced fluorescence (ps-TALIF) in krypton: Preventing saturation regimes for reliable calibration of atomic densities. Phys. Plasmas 2020. Submitted on 23 December 2020. Available online: https://hal.archives-ouvertes.fr/hal-03087285v1 (accessed on 29 December 2020).
- Miyazaki, K.; Kajiwara, T.; Uchino, K.; Muraoka, K.; Okada, T.; Maeda, M. Laser-induced dissociation of molecules during measurements of hydrogen atoms in processing plasmas using two-photon laser-induced fluorescence. J. Vac. Sci. Technol. A 1996, 14, 125–131. [Google Scholar] [CrossRef]
- Reuter, S.; Niemi, K.; Schulz-von der Gathen, V.; Döbele, H.F. Generation of atomic oxygen in the effluent of an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2009, 18, 015006. [Google Scholar] [CrossRef]
- Es-Sebbar, E.-T.; Sarra-Bournet, C.; Naudé, N.; Massines, F.; Gherardi, N. Absolute nitrogen atom density measurements by two-photon laser-induced fluorescence spectroscopy in atmospheric pressure dielectric barrier discharges of pure nitrogen. J. Appl. Phys. 2009, 106, 073302. [Google Scholar] [CrossRef]
- Teramoto, Y.; Ono, R.; Oda, T. Production mechanism of atomic nitrogen in atmospheric pressure pulsed corona discharge measured using two-photon absorption laser-induced fluorescence. J. Appl. Phys. 2012, 111, 113302. [Google Scholar] [CrossRef]
- Zhang, S.; van Gessel, A.F.H.; van Grootel, S.C.; Bruggeman, P.J. The effect of collisional quenching of the O3p3PJ state on the determination of the spatial distribution of the atomic oxygen density in an APPJ operating in ambient air by TALIF. Plasma Sources Sci. Technol. 2014, 23, 025012. [Google Scholar] [CrossRef] [Green Version]
- Bergano, N.S.; Bechtel, J.H.; Jaanimagi, P.A.; Salour, M.M. Picosecond laser-spectroscopy measurement of hydroxyl fluorescence lifetime in flames. Opt. Lett. 1983, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Capps, C.; Kulatilaka, W.D. Femtosecond two-photon laser-induced fluorescence of krypton for high-speed flow imaging. Opt. Lett. 2017, 42, 711. [Google Scholar] [CrossRef] [PubMed]
- West, A.; Bredin, J.; Schröter, S.; Niemi, K.; Dedrick, J.; O’Connell, D.; Gans, T.; Wagenaars, E. Picosecond two-photon absorption laser induced fluorescence for measuring reactive atomic species in atmospheric-prtessure plasma jets. In Proceedings of the 19th International Conference on Atomic Processes in Plasmas (APiP), Paris, France, 4–8 April 2016. [Google Scholar]
- Conway, J.; Gogna, G.S.; Gaman, C.; Turner, M.M.; Daniels, S. Two-photon absorption laser induced fluorescence measurement of atomic oxygen density in an atmospheric pressure air plasma jet. Plasma Sources Sci. Technol. 2016, 25, 045023. [Google Scholar] [CrossRef] [Green Version]
- Brisset, A.; Gazeli, K.; Magne, L.; Pasquiers, S.; Jeanney, P.; Marode, E.; Tardiveau, P. Modification of the electric field distribution in a diffuse streamer-induced discharge under extreme overvoltage. Plasma Sources Sci. Technol. 2019, 28, 055016. [Google Scholar] [CrossRef]
- Goldsmith, J.E.M.; Aldén, M.; Westblom, U. Two-photon-excited stimulated emission from atomic oxygen in flames and cold gases. Opt. Lett. 1989, 14, 305. [Google Scholar]
- Alekseev, V.; Setser, D.W. Quenching rate constants and product assignments for reactions of Xe(7p[3/2]2, 7p[5/2]2, and 6p‘[3/2]2) atoms with rare gases, CO, H2, N2O, CH4, and halogen-containing molecules. J. Phys. Chem. 1996, 100, 5766–5780. [Google Scholar] [CrossRef]
- Mazouffre, S.; Foissac, C.; Supiot, P.; Vankan, P.; Engeln, R.; Schram, D.C.; Sadeghi, N. Density and temperature of N atoms in the afterglow of a microwave discharge measured by a two-photon laser-induced fluorescence technique. Plasma Sources Sci. Technol. 2001, 10, 168–175. [Google Scholar] [CrossRef]
- Gaboriau, F.; Cvelbar, U.; Mozetic, M.; Erradi, A.; Rouffet, B. Comparison of TALIF and catalytic probes for the determination of nitrogen atom density in a nitrogen plasma afterglow. J. Phys. D Appl. Phys. 2009, 42, 055204. [Google Scholar] [CrossRef]





| Quantities | ||||
|---|---|---|---|---|
| Atom-Excited State | N2 | O2 | ||
| Ν: (3p)4 | = 0.67 50% [36] | 29.6 [36] | 0.41 [36] | 6.63 [36] |
| H: 3d2D3/2,5/2 | = 0.62 50% [36] | 17.6 [36] | 20.1 [36] | 32.6 [36] |
| O: 3p3P1,2,0 | = 0.36 50% [36] = 0.51 50% [92] (for Xe: 7p [3/2]2) | 35.1 [36] 34.7 1.7 [78] | 5.9 [36] | 9.3 [36] |
| = 1.9 ( 20%) [78] (for Xe: 6p’[3/2]2) | ||||
| Kr: 5p’[3/2]2 | = 0.67 50% [36] = 0.62 50% [36] | 34.1 [36] | 3.35[36] | 6.64[36] |
| Xe: 7p[3/2]2 | = 0.36 50% [36] = 0.51 50% [92] | 105.6 [36] | 14 [36] | 20.6 [36] |
| Xe: 6p’[3/2]2 | = 1.9 ( 20%) [78] | 40.8 2 [78] | 9.4 0.5 [36] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazeli, K.; Lombardi, G.; Aubert, X.; Duluard, C.Y.; Prasanna, S.; Hassouni, K. Progresses on the Use of Two-Photon Absorption Laser Induced Fluorescence (TALIF) Diagnostics for Measuring Absolute Atomic Densities in Plasmas and Flames. Plasma 2021, 4, 145-171. https://0-doi-org.brum.beds.ac.uk/10.3390/plasma4010009
Gazeli K, Lombardi G, Aubert X, Duluard CY, Prasanna S, Hassouni K. Progresses on the Use of Two-Photon Absorption Laser Induced Fluorescence (TALIF) Diagnostics for Measuring Absolute Atomic Densities in Plasmas and Flames. Plasma. 2021; 4(1):145-171. https://0-doi-org.brum.beds.ac.uk/10.3390/plasma4010009
Chicago/Turabian StyleGazeli, Kristaq, Guillaume Lombardi, Xavier Aubert, Corinne Y. Duluard, Swaminathan Prasanna, and Khaled Hassouni. 2021. "Progresses on the Use of Two-Photon Absorption Laser Induced Fluorescence (TALIF) Diagnostics for Measuring Absolute Atomic Densities in Plasmas and Flames" Plasma 4, no. 1: 145-171. https://0-doi-org.brum.beds.ac.uk/10.3390/plasma4010009