Inflammatory Cytokines Facilitate the Sensitivity of P2X7 Receptors Toward Extracellular ATP at Neural Progenitor Cells of the Rodent Hippocampal Subgranular Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hippocampal Brain Slices and the Corresponding Organotypic Slice Cultures
2.2. Whole-Cell Patch-Clamp Recordings
2.3. Drug Application Protocols
2.4. Multiple Immunofluorescence Labeling Under Confocal Microscopic Observation
2.5. Materials
2.6. Statistics
3. Results
3.1. Sensitivity Increase of P2X7Rs at SGZ NPCs in Organotypic Hippocampal Slices Caused by Pre-Incubation with Lipopolysaccharide and Cytokines
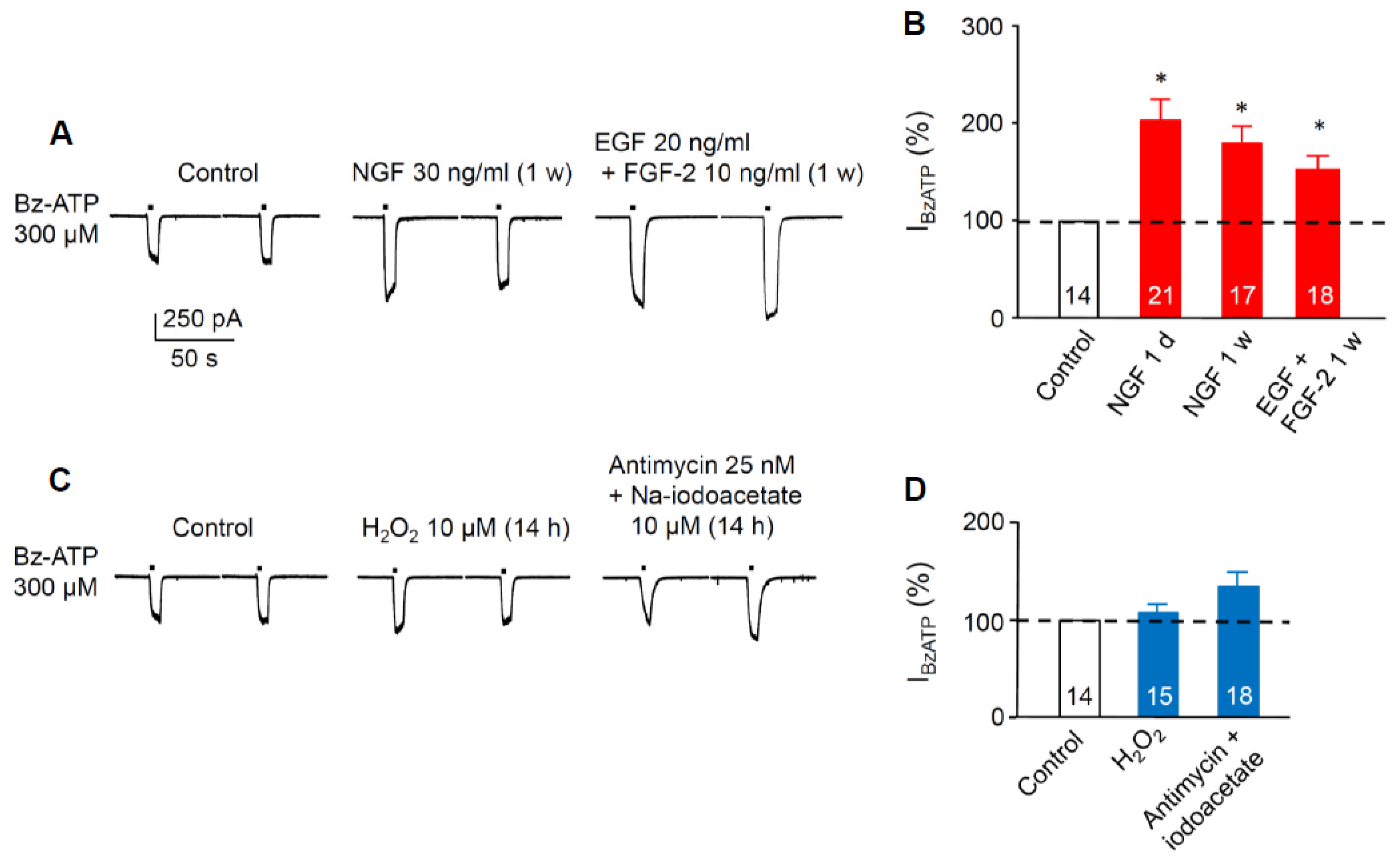
3.2. Sensitivity Increase of P2X7Rs at SGZ NPCs in Organotypic Hippocampal Slices Caused Pre-Incubation with Growth Factors, but not by Pre-Incubation with Reactive Oxygen Species or Metabolic Inhibitors
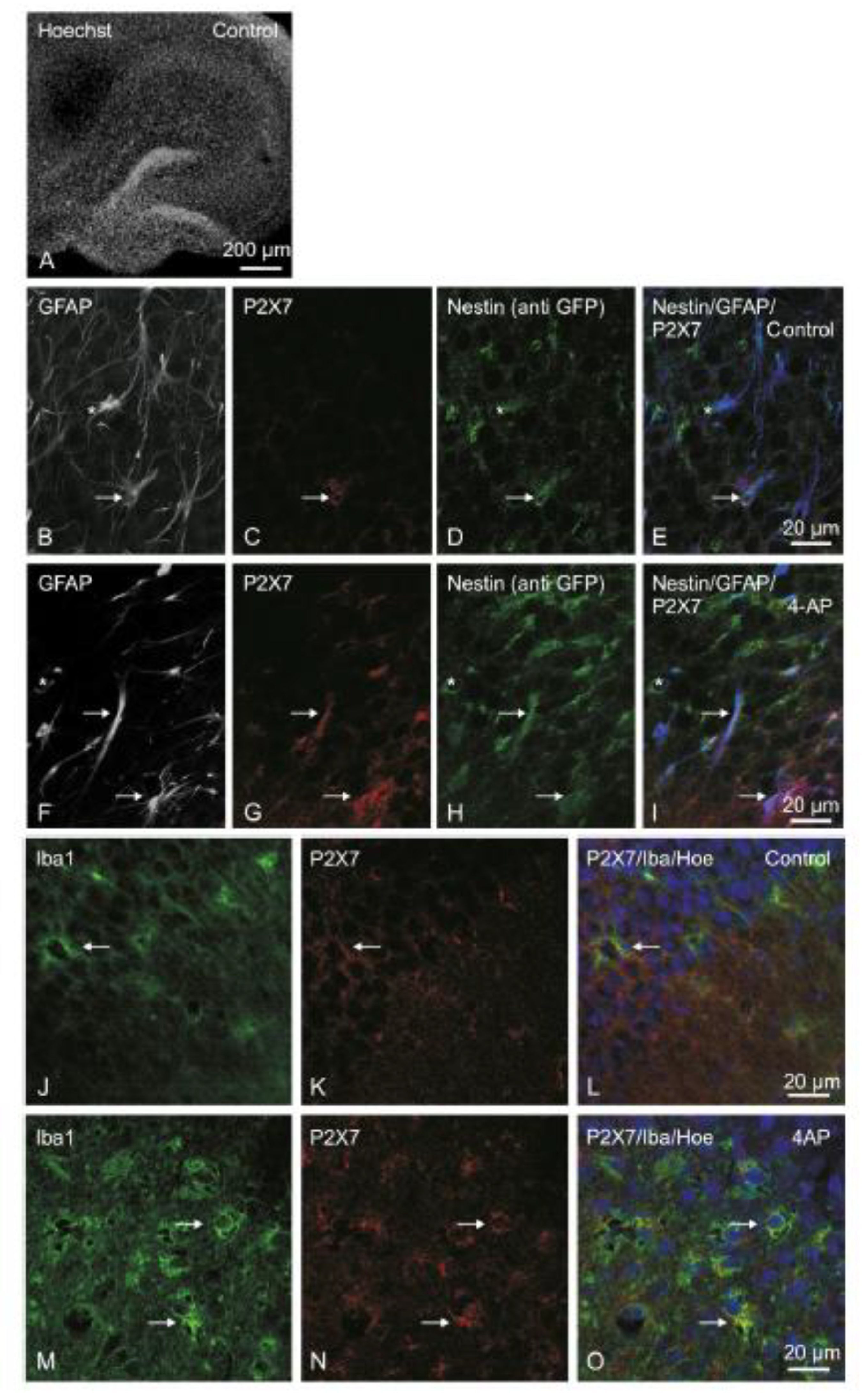
3.3. P2X7R Immunoreactivity in Organotypic Hippocampal Slice Cultures
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jessberger, S.; Gage, F.H. Adult neurogenesis: Bridging the gap between mice and humans. Trends Cell. Biol. 2014, 24, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Illes, P. Regulation of adult neural progenitor cell functions by purinergic signaling. Glia 2017, 65, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Illes, P.; Ulrich, H. Purinergic receptors in embryonic and adult neurogenesis. Neuropharmacology 2016, 104, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.M.; Jessberger, S. Adult neurogenesis: Mechanisms and functional significance. Development 2014, 141, 1983–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, N. Molecular Mechanism of Adult Neurogenesis and its Association with Human Brain Diseases. J. Cent. Nerv. Syst. Dis. 2016, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Parent, J.M. Epilepsy and Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7, a020677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperlagh, B.; Illes, P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illes, P.; Khan, T.M.; Rubini, P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J. Neurosci. 2017, 37, 7049–7062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, J.A.; Young, M.T.; Sung, H.Y.; North, R.A.; Surprenant, A. Reanalysis of P2X7 receptor expression in rodent brain. J. Neurosci. 2004, 24, 6307–6314. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Beamer, E.; Goloncser, F.; Horvath, G.; Beko, K.; Otrokocsi, L.; Kovanyi, B.; Sperlagh, B. Purinergic mechanisms in neuroinflammation: An update from molecules to behavior. Neuropharmacology 2016, 104, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Lemoli, R.M.; Curti, A.; Idzko, M.; Panther, E.; Di Virgilio, F. The P2X7 receptor: A key player in IL-1 processing and release. J. Immunol. 2006, 176, 3877–3883. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.L.; Sarti, A.C.; Falzoni, S.; Di Virgilio, F. The P2X7 Receptor-Interleukin-1 Liaison. Front. Pharmacol. 2017, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.J.; Gorecki, D.C. P2RX7 Purinoceptor as a Therapeutic Target-The Second Coming? Front. Chem. 2018, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Dal, B.D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Rozmer, K.; Gao, P.; Araujo, M.G.L.; Khan, M.T.; Liu, J.; Rong, W.; Tang, Y.; Franke, H.; Krugel, U.; Fernandes, M.J.S.; et al. Pilocarpine-Induced Status Epilepticus Increases the Sensitivity of P2X7 and P2Y1 Receptors to Nucleotides at Neural Progenitor Cells of the Juvenile Rodent Hippocampus. Cereb. Cortex 2017, 27, 3568–3585. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Liu, J.; Tang, Y.; Illes, P. Regulation of P2X7 receptor function of neural progenitor cells in the hippocampal subgranular zone by neuronal activity in the dentate gyrus. Neuropharmacology 2018, 140, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Heine, C.; Servettini, I.; Gullo, F.; Sygnecka, K.; Franke, H.; Illes, P.; Wanke, E. Functional regeneration of the ex-vivo reconstructed mesocorticolimbic dopaminergic system. Cereb. Cortex 2013, 23, 2905–2922. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ding, X.; Khan, T.M.; Rong, W.; Franke, H.; Illes, P. P2X7 receptor-sensitivity of astrocytes and neurons in the substantia gelatinosa of organotypic spinal cord slices of the mouse depends on the length of the culture period. Neuroscience 2017, 349, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Riedel, T.; Leichsenring, A.; Heine, C.; Franke, H.; Krugel, U.; Norenberg, W.; Illes, P. Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb. Cortex 2011, 21, 806–820. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Domercq, M.; Matute, C. Inflammation in stroke: The role of cholinergic, purinergic and glutamatergic signaling. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418774267. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A. Epilepsy and inflammation in the brain: Overview and pathophysiology. Epilepsy Curr. 2014, 14, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.M.; Sun, M.; Crack, P.; O’Brien, T.J.; Shultz, S.R.; Semple, B.D. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflammation 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, A.; Riedel, T.; Qin, Y.; Rubini, P.; Illes, P. Anoxic depolarization of hippocampal astrocytes: Possible modulation by P2X7 receptors. Neurochem. Int. 2013, 62, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Pelegrin, P.; Surprenant, A. Facilitation of P2X7 receptor currernts and membrane blebbing via constitutive and dynamic calmodulin binding. J. Neurosci. 2008, 28, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Probert, L.; Akassoglou, K.; Pasparakis, M.; Kontogeorgos, G.; Kollias, G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 1995, 92, 11294–11298. [Google Scholar] [CrossRef] [PubMed]
- Godukhin, O.V.; Levin, S.G.; Parnyshkova, E.Y. The effects of interleukin-10 on the development of epileptiform activity in the hippocampus induced by transient hypoxia, bicuculline, and electrical kindling. Neurosci. Behav. Physiol. 2009, 39, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Galic, M.A.; Riazi, K.; Heida, J.G.; Mouihate, A.; Fournier, N.M.; Spencer, S.J.; Kalynchuk, L.E.; Teskey, G.C.; Pittman, Q.J. Postnatal inflammation increases seizure susceptibility in adult rats. J. Neurosci. 2008, 28, 6904–6913. [Google Scholar] [CrossRef] [PubMed]
- Harre, E.M.; Galic, M.A.; Mouihate, A.; Noorbakhsh, F.; Pittman, Q.J. Neonatal inflammation produces selective behavioural deficits and alters N-methyl-d-aspartate receptor subunit mRNA in the adult rat brain. Eur. J. Neurosci. 2008, 27, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Khadra, A.; Sherman, A.; Stojilkovic, S.S. Calcium-dependent block of P2X7 receptor channel function is allosteric. J. Gen. Physiol. 2011, 138, 437–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leschinger, A.; Stabel, J.; Igelmund, P.; Heinemann, U. Pharmacological and electrographic properties of epileptiform activity induced by elevated K+ and lowered Ca2+ and Mg2+ concentration in rat hippocampal slices. Exp. Brain. Res. 1993, 96, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Illes, P. Nucleotide signaling in astrogliosis. Neurosci. Lett. 2014, 565, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Illes, P. Pathological potential of astroglial purinergic receptors. Adv. Neurobiol. 2014, 11, 213–256. [Google Scholar] [PubMed]
- Franke, H.; Verkhratsky, A.; Burnstock, G.; Illes, P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012, 8, 629–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, H.; Günther, A.; Grosche, J.; Schmidt, R.; Rossner, S.; Reinhardt, R.; Faber-Zuschratter, H.; Schneider, D.; Illes, P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J. Neuropathol. Exp. Neurol. 2004, 63, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.Y.; Li, A.P. P2X7 receptors in cerebral ischemia. Neurosci. Bull. 2013, 29, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melani, A.; Amadio, S.; Gianfriddo, M.; Vannucchi, M.G.; Volonte, C.; Bernardi, G.; Pedata, F.; Sancesario, G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J. Cereb. Blood Flow Metab. 2006, 26, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Penk, A.; Franke, H.; Krugel, U.; Norenberg, W.; Huster, D.; Schaefer, M. Lack of functional P2X7 receptor aggravates brain edema development after middle cerebral artery occlusion. Purinergic Signal. 2016, 12, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Guo, Z.; Liu, X.; Ouyang, Q.; He, C.; Burnstock, G.; Yuan, H.; Xiang, Z. Block of P2X7 receptors could partly reverse the delayed neuronal death in area CA1 of the hippocampus after transient global cerebral ischemia. Purinergic Signal. 2013, 9, 663–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, K.; Yin, B.; Wang, J.; Peng, G.; Liang, H.; Xu, Z.; Du, Y.; Fang, M.; Xia, Q.; Luo, B. Inhibition of P2X7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J. Neuroinflammation 2012, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirkner, K.; Kofalvi, A.; Fischer, W.; Gunther, A.; Franke, H.; Groger-Arndt, H.; Norenberg, W.; Madarasz, E.; Vizi, E.S.; Schneider, D.; et al. Supersensitivity of P2X receptors in cerebrocortical cell cultures after in vitro ischemia. J. Neurochem. 2005, 95, 1421–1437. [Google Scholar] [CrossRef] [PubMed]
- Dubé, C.M.; Brewster, A.L.; Richichi, C.; Zha, Q.; Baram, T.Z. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Khan, M.T.; Tang, Y.; Franke, H.; Illes, P. Inflammatory Cytokines Facilitate the Sensitivity of P2X7 Receptors Toward Extracellular ATP at Neural Progenitor Cells of the Rodent Hippocampal Subgranular Zone. Neuroglia 2018, 1, 258-270. https://0-doi-org.brum.beds.ac.uk/10.3390/neuroglia1010017
Liu J, Khan MT, Tang Y, Franke H, Illes P. Inflammatory Cytokines Facilitate the Sensitivity of P2X7 Receptors Toward Extracellular ATP at Neural Progenitor Cells of the Rodent Hippocampal Subgranular Zone. Neuroglia. 2018; 1(1):258-270. https://0-doi-org.brum.beds.ac.uk/10.3390/neuroglia1010017
Chicago/Turabian StyleLiu, Juan, Muhammad Tahir Khan, Yong Tang, Heike Franke, and Peter Illes. 2018. "Inflammatory Cytokines Facilitate the Sensitivity of P2X7 Receptors Toward Extracellular ATP at Neural Progenitor Cells of the Rodent Hippocampal Subgranular Zone" Neuroglia 1, no. 1: 258-270. https://0-doi-org.brum.beds.ac.uk/10.3390/neuroglia1010017




