Characteristics and Clinical Outcomes of Individuals at High Risk for Pancreatic Cancer: A Descriptive Analysis from a Comprehensive Cancer Center
Abstract
:1. Introduction
1.1. Risk Factors for PC
1.2. Pre-Malignant PC Precursors Exist and Can Be Detected via Imaging
2. Results
2.1. Characteristics of the Study Cohort
2.2. Outcomes of HRI that met CAPS Guidelines
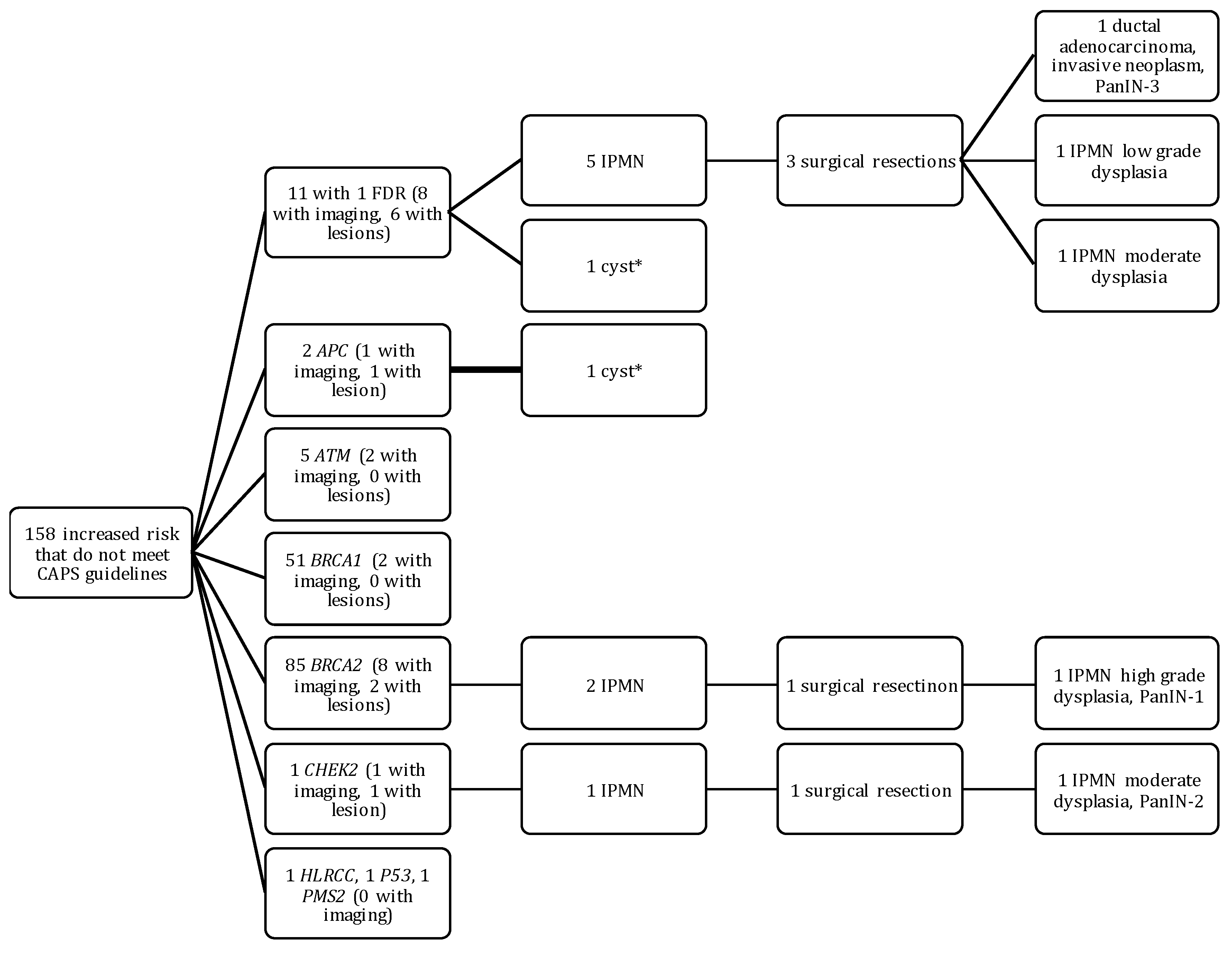
2.3. Outcomes of HRI That Did Not Meet CAPS Guidelines
2.4. Outcomes of Sporadic Cases
3. Discussion
4. Methods
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Noone, A.M.H.N.; Krapcho, M.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; Feuer, E.J.; et al. SEER Cancer Statistics Review, 1975–2015. Available online: https://seer.cancer.gov/csr/1975_2015/ (accessed on 20 July 2018).
- Shi, C.; Hruban, R.H.; Klein, A.P. Familial pancreatic cancer. Arch. Pathol. Lab. Med. 2009, 133, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA A Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Gangi, A.; Malafa, M.; Klapman, J. Endoscopic Ultrasound–Based Pancreatic Cancer Screening of High-Risk Individuals: A Prospective Observational Trial. Pancreas 2018, 47, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.-W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013, 62, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xu, C.-F.; Wan, X.-Y.; Zhu, H.-T.; Yu, C.-H.; Li, Y.-M. Screening for pancreatic cancer in familial high-risk individuals: A systematic review. World J. Gastroenterol. WJG 2015, 21, 8678–8686. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA A Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Bondy, M.L.; Wolff, R.A.; Abbruzzese, J.L.; Vauthey, J.-N.; Pisters, P.W.; Evans, D.B.; Khan, R.; Chou, T.-H.; Lenzi, R.; et al. Risk Factors for Pancreatic Cancer: Case-Control Study. Am. J. Gastroenterol. 2007, 102, 2696. [Google Scholar] [CrossRef] [PubMed]
- Permuth-Wey, J.; Egan, K.M. Family history is a significant risk factor for pancreatic cancer: Results from a systematic review and meta-analysis. Fam. Cancer 2009, 8, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P.; Brune, K.A.; Petersen, G.M.; Goggins, M.; Tersmette, A.C.; Offerhaus, G.J.A.; Griffin, C.; Cameron, J.L.; Yeo, C.J.; Kern, S.; et al. Prospective Risk of Pancreatic Cancer in Familial Pancreatic Cancer Kindreds. Cancer Res. 2004, 64, 2634–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz-Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, D.K.; Gress, T.M.; Langer, P. Familial pancreatic cancer—Current knowledge. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Klein, A.P.; Goggins, M.; Maitra, A.; Canto, M.; Ali, S.; Schulick, R.; Palmisano, E.; Hruban, R.H. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin. Cancer Res. 2009, 15, 7737–7743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartsch, D.K.; Slater, E.P.; Carrato, A.; Ibrahim, I.S.; Guillen-Ponce, C.; Vasen, H.F.; Matthai, E.; Earl, J.; Jendryschek, F.S.; Figiel, J.; et al. Refinement of screening for familial pancreatic cancer. Gut 2016, 65, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Easton, D.F.; The Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 Mutation Carriers. JNCI J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potjer, T.P.; Schot, I.; Langer, P.; Heverhagen, J.T.; Wasser, M.N.J.M.; Slater, E.P.; Klöppel, G.; Morreau, H.M.; Bonsing, B.A.; de Vos tot Nederveen Cappel, W.H.; et al. Variation in Precursor Lesions of Pancreatic Cancer among High-Risk Groups. Clin. Cancer Res. 2013, 19, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.L.; Shakya, R.; Lipsyc, M.D.; Mitchel, E.B.; Kumar, S.; Hwang, C.; Deng, L.; Devoe, C.; Chabot, J.A.; Szabolcs, M.; et al. High Prevalence of BRCA1 and BRCA2 Germline Mutations with Loss of Heterozygosity in a Series of Resected Pancreatic Adenocarcinoma and Other Neoplastic Lesions. Clin. Cancer Res. 2013, 19, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- The Breast Cancer Linkage Consortium. Cancer Risks in BRCA2 Mutation Carriers. JNCI J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Van Asperen, C.J.; Brohet, R.M.; Meijers-Heijboer, E.J.; Hoogerbrugge, N.; Verhoef, S.; Vasen, H.F.A.; Ausems, M.G.E.M.; Menko, F.H.; Gomez Garcia, E.B.; Klijn, J.G.M.; et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J. Med. Genet. 2005, 42, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubezky, N.; Ben-Haim, M.; Lahat, G.; Marmor, S.; Solar, I.; Brazowski, E.; Nackache, R.; Klausner, J.M. Intraductal papillary mucinous neoplasm of the pancreas: Associated cancers, family history, genetic predisposition? Surgery 2012, 151, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Rosty, C.; Jansen, M.; Fukushima, N.; Ueki, T.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Goggins, M. STK11/LKB1 Peutz-Jeghers Gene Inactivation in Intraductal Papillary-Mucinous Neoplasms of the Pancreas. Am. J. Pathol. 2001, 159, 2017–2022. [Google Scholar] [CrossRef] [Green Version]
- Vasen, H.F.A.; Wasser, M.; van Mil, A.; Tollenaar, R.A.; Konstantinovski, M.; Gruis, N.A.; Bergman, W.; Hes, F.J.; Hommes, D.W.; Offerhaus, G.J.A.; et al. Magnetic Resonance Imaging Surveillance Detects Early-Stage Pancreatic Cancer in Carriers of a p16-Leiden Mutation. Gastroenterology 2011, 140, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Sparr, J.A.; Bandipalliam, P.; Redston, M.S.; Syngal, S. Intraductal papillary mucinous neoplasm of the pancreas with loss of mismatch repair in a patient with Lynch syndrome. Am. J. Surg. Pathol. 2009, 33, 309. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-Y. Intraductal Papillary Mucinous Neoplasm of the Pancreas: An Update. Scientifica 2012, 2012, 20. [Google Scholar] [CrossRef] [PubMed]
- Chetty, R.; Salahshor, S.; Bapat, B.; Berk, T.; Croitoru, M.; Gallinger, S. Intraductal papillary mucinous neoplasm of the pancreas in a patient with attenuated familial adenomatous polyposis. J. Clin. Pathol. 2005, 58, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sessa, F.; Solcia, E.; Capella, C.; Bonato, M.; Scarpa, A.; Zamboni, G.; Pellegata, N.S.; Ranzani, G.N.; Rickaert, F.; Klöppel, G. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: An investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994, 425, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Talamini, G.; Zamboni, G.; Salvia, R.; Capelli, P.; Sartori, N.; Casetti, L.; Bovo, P.; Vaona, B.; Falconi, M.; Bassi, C. Intraductal papillary mucinous neoplasms and chronic pancreatitis. Pancreatology 2006, 6, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, D.; Saviano, A.; Serricchio, M.; Dal Lago, A.; Brizi, M.; Manfredi, R.; Costamagna, G.; Attili, F.J.E. The association of pancreatic cystosis and IPMN in cystic fibrosis: Case report and literature review. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5179–5184. [Google Scholar] [PubMed]
- Yonezawa, S.; Higashi, M.; Yamada, N.; Goto, M. Precursor lesions of pancreatic cancer. Gut Liver 2008, 2, 137. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Adsay, N.V.; Albores-Saavedra, J.; Compton, C.; Garrett, E.S.; Goodman, S.N.; Kern, S.E.; Klimstra, D.S.; Kloppel, G.; Longnecker, D.S.; et al. Pancreatic intraepithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol. 2001, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Maitra, A.; Kern, S.E.; Goggins, M. Precursors to Pancreatic Cancer. Gastroenterol. Clin. N. Am. 2007, 36, 831–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruban, R.H.; Takaori, K.; Klimstra, D.S.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Biankin, S.A.; Compton, C.; Fukushima, N.; Furukawa, T.; et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 2004, 28, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, H.; Schulick, R.D.; Hruban, R.H.; Maitra, A. Cystic precursors to invasive pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, G.; Hirabayashi, K.; Castelli, P.; Lennon, A.M. Precancerous lesions of the pancreas. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Goggins, M.; Hruban, R.H.; Petersen, G.M.; Giardiello, F.M.; Yeo, C.; Fishman, E.K.; Brune, K.; Axilbund, J.; Griffin, C.; et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin. Gastroenterol. Hepatol. 2006, 4, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Torphy, R.J.; Schulick, R.D. Screening of Patients at Risk for Familial Pancreatic Cancer. Surg. Clin. 2018, 98, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernandez-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Hwang, C.; Stevens, P.D.; Rotterdam, H.; Stavropoulos, S.N.; Sy, C.D.; Prince, M.A.; Chung, W.K.; Fine, R.L.; Chabot, J.A.; et al. Pancreatic Cancer Screening in a Prospective Cohort of High-Risk Patients: A Comprehensive Strategy of Imaging and Genetics. Clin. Cancer Res. 2010, 16, 5028–5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, R.E.; Lerch, M.M.; Rubinstein, W.S.; Neoptolemos, J.P.; Whitcomb, D.C.; Hruban, R.H.; Brentnall, T.A.; Lynch, H.T.; Canto, M.I. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut 2007, 56, 1460–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018. [Google Scholar] [CrossRef] [PubMed]
- Kimmey, M.B.; Bronner, M.P.; Byrd, D.R.; Brentnall, T.A. Screening and surveillance for hereditary pancreatic cancer. Gastrointest. Endosc. 2002, 56, S82–S86. [Google Scholar] [CrossRef]
- Özcelik, H.; Schmocker, B.; Nicola, N.D.; Shi, X.-H.; Langer, B.; Moore, M.; Taylor, B.R.; Narod, S.A.; Darlington, G.; Andrulis, I.L.; et al. Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat. Genet. 1997, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of Incidental Pancreatic Cysts in the Adult Population on MR Imaging. Am. J. Gastroenterol. 2010, 105, 2079. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Scheiman, J.; Anderson, M.A.; Hines, O.J.; Reber, H.A.; Farrell, J.; Kochman, M.L.; Foley, P.J.; Drebin, J.; Oh, Y.S.; et al. Risk of Malignancy in Resected Cystic Tumors of the Pancreas ≤3 cm in Size: Is it Safe to Observe Asymptomatic Patients? A Multi-institutional Report. J. Gastrointest. Surg. 2008, 12, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Bamlet, W.R.; Moore, R.M.; Nandakumar, K.; Eckloff, B.W.; Lee, Y.K.; Petersen, G.M.; McWilliams, R.R.; Couch, F.J.J.C.E.; et al. Prevalence of pathogenic mutations in cancer predisposition genes among pancreatic cancer patients. Cancer Epidemiol. Prev. Biomark. 2016, 25, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Permuth, J.B.; Trevino, J.; Merchant, N.; Malafa, M.; Florida Pancreas Collaborative. Partnering to advance early detection and prevention efforts for pancreatic cancer: The Florida Pancreas Collaborative. Future Med. 2016, 12, 997–1000. [Google Scholar] [CrossRef] [PubMed]


| Syndrome | Gene(s) | Risk of PC by Age 70–75 | Studies Reporting PC Precursor Lesions * among Carriers |
|---|---|---|---|
| Hereditary Breast and Ovarian Cancer (HBOC) | BRCA2 | 4.5–8% | [23] |
| BRCA1, PALB2 | 3.6% | ||
| Peutz-Jeghers (PJS) | STK11/LKB1 | 36% | [24] |
| Familial atypical multiple-mole melanoma (FAMMM) | P16/CDKN2A | 13–17% | [25] |
| Hereditary non-polyposis colorectal cancer (HNPCC) | MSH2, MLH1, MSH6, PMS1, PMS2 | 3.7% | [23,26,27] |
| Familial adenomatous polyposis (FAP) | APC | 1.7% | [28] |
| Ataxia telangiectasia | ATM | <5% | None identified |
| Li Fraumeni | TP53 | <5% | [29] |
| Hereditary Pancreatitis | PRSS1, SPINK1 | 25–54% | [30] |
| Cystic Fibrosis | CFTR | <5% | [31] |
| Characteristic | Entire Cohort | High Risk According to CAPS Guidelines (n = 105) | Increased risk that Does Not Meet CAPS Guidelines (n = 158) | Sporadic Cases (n = 66) | p * |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 86 (26.1) | 33 (31.4) | 21 (13.3) | 32 (48.5) | <0.0001 |
| Female | 243 (73.9) | 72 (68.6) | 137 (86.7) | 34 (51.5) | |
| Race/ethnicity | |||||
| Non-Hispanic White | 277 (84.5) | 93 (88.5) | 127 (80.9) | 57 (86.4) | 0.86 |
| Non-Hispanic Black | 18 (5.5) | 5 (4.8) | 10 (6.4) | 3 (4.5) | |
| Hispanic White | 22 (6.7) | 5 (4.8) | 13 (8.3) | 4 (6.1) | |
| Other | 11 (3.3) | 2 (1.9) | 7(4.4) | 2 (3.0) | |
| Missing | 1 | 0 | 0 | 1 | |
| Smoking Status | |||||
| Current | 27 (8.2) | 9 (8.9) | 14 (10.2) | 4 (6.3) | 0.06 |
| Former | 192 (58.4) | 23 (22.8) | 33 (23.9) | 27 (42.9) | |
| Never | 83 (25.2) | 69 (68.3) | 91 (65.9) | 32 (50.8) | |
| Missing | 27 | 4 | 20 | 3 | |
| Personal History of Cancer or Other Conditions | |||||
| Breast Cancer | 10 (3.1) | 5 (4.8) | 2 (1.3) | 3 (4.5) | € |
| Colorectal cancer | 3 (0.9) | 0 (0.0) | 1(0.6) | 2 93.0) | |
| ¥ Gynecological Cancer | 3 (0.9) | 1 (1.0) | 0 (0.0) | 2 (3.0) | |
| Prostate Cancer | 10 (3.1) | 2 (1.9) | 0 (0.0) | 8 (12.1) | |
| ¥ Other cancer | 13 (4.0) | 1 (1.0) | 2 (1.3) | 10 (15.2) | |
| Pancreatitis | 9 (2.8) | 2 (1.9) | 2 (1.3) | 5 (7.6) | |
| Diabetes | 9 (2.8) | 1 (1.0) | 1 (0.6) | 7 (10.6) | |
| None | 270 (82.6) | 92 (88.5) | 149 (94.9) | 29 (43.9) | |
| Missing | 2 | 1 | 1 | 0 | |
| Pancreatic lesion detected on imaging | |||||
| Had imaging and a lesion was detected | 107 (32.5) | 31 (29.5) | 10 (6.3) | 66 (100.0) | <0.0001 |
| Had imaging and no lesion was detected | 65 (19.8) | 52 (49.5) | 13 (8.2) | 0 (0.0) | |
| No imaging on file | 157 (47.7) | 22 (21.0) | 135 (85.4) | 0 (0.0) | |
| Type of pancreatic lesion detected | <0.001 † | ||||
| Cyst, type not specified | 49 (14.9) | 20 (19.1) | 2 (1.3) | 27 (40.9) | |
| Intraductal papillary mucinous neoplasm | 51 (15.5) | 9 (8.6) | 8 (5.1) | 34 (51.5) | |
| Mucinous cystic neoplasm | 1 (0.3) | 0 (0.0)) | 0 (0.0) | 1 (1.5) | |
| Pseudocyst | 2 (0.6) | 0 (0.0) | 0 (0.0) | 2 (3.0) | |
| Solid Tumor/Adenocarcinoma | 3 (0.9) | 2 (1.9) | 0 (0.0) | 1 (1.5) | |
| Pancreatic Neuroendocrine Tumor | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (1.5) | |
| Average age at diagnosis of pancreatic lesion | 65.5 | 62.6 | 60.7 | 67.6 | <0.020 |
| Average size of largest lesion detected (cm) | 2 | 0.7 | 2 | 2.6 | <0.0001 |
| Body Mass Index (BMI) | - | 28.3 | 30.3 | 27 | 0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNamara, G.P.J.; Ali, K.N.; Vyas, S.; Huynh, T.; Nyland, M.; Almanza, D.; Laronga, C.; Klapman, J.; Permuth, J.B. Characteristics and Clinical Outcomes of Individuals at High Risk for Pancreatic Cancer: A Descriptive Analysis from a Comprehensive Cancer Center. Gastrointest. Disord. 2019, 1, 106-119. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1010008
McNamara GPJ, Ali KN, Vyas S, Huynh T, Nyland M, Almanza D, Laronga C, Klapman J, Permuth JB. Characteristics and Clinical Outcomes of Individuals at High Risk for Pancreatic Cancer: A Descriptive Analysis from a Comprehensive Cancer Center. Gastrointestinal Disorders. 2019; 1(1):106-119. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1010008
Chicago/Turabian StyleMcNamara, Griffin P. J., Karla N. Ali, Shraddha Vyas, Tri Huynh, Monica Nyland, Deanna Almanza, Christine Laronga, Jason Klapman, and Jennifer B. Permuth. 2019. "Characteristics and Clinical Outcomes of Individuals at High Risk for Pancreatic Cancer: A Descriptive Analysis from a Comprehensive Cancer Center" Gastrointestinal Disorders 1, no. 1: 106-119. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1010008





