Corticosteroid Injection Alone or Combined with Surgical Excision of Keloids versus Other Therapies Including Ionising Radiotherapy: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
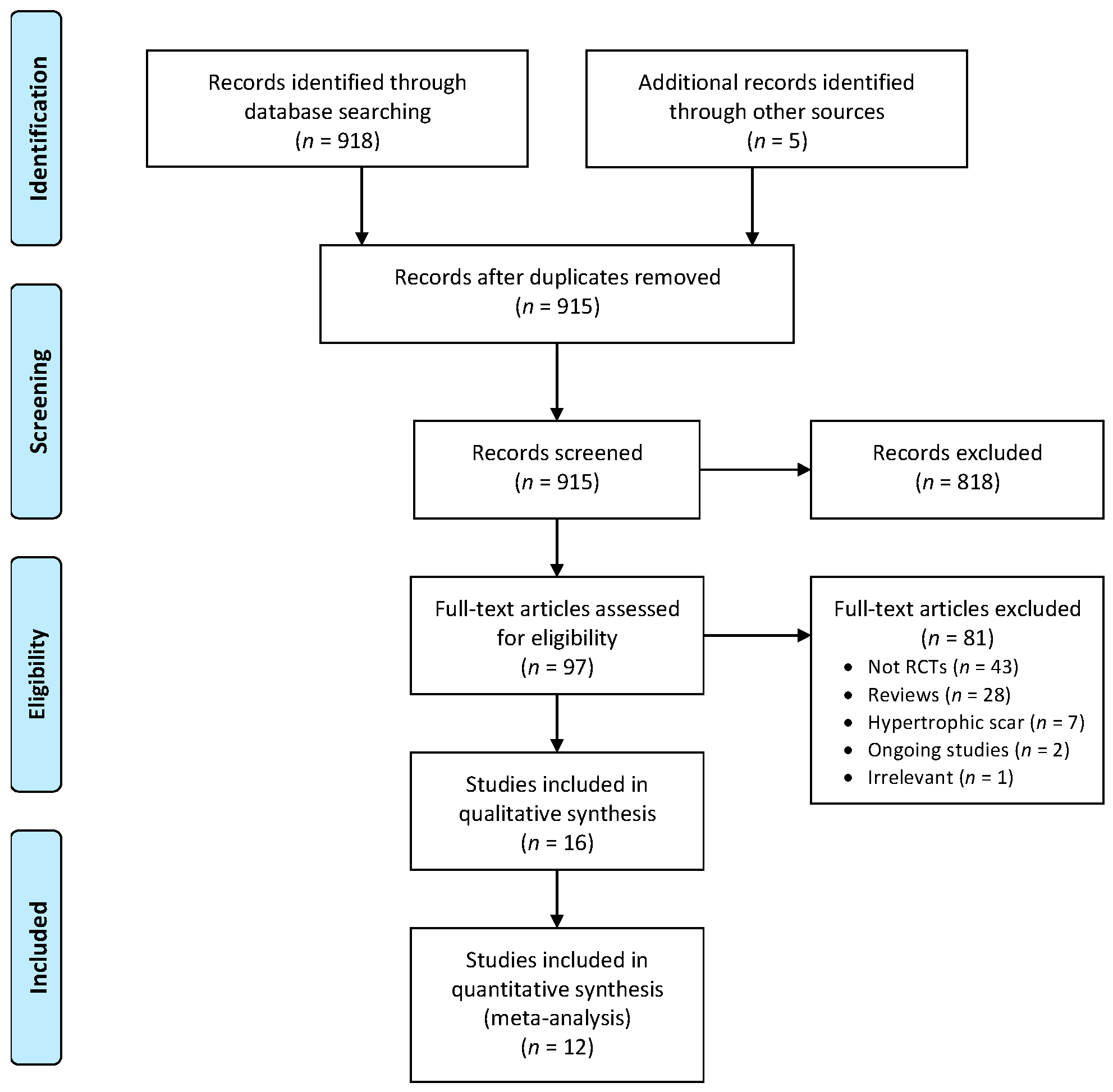
2. Materials and Methods
2.1. Search Strategy
2.2. Data Collection and Analysis
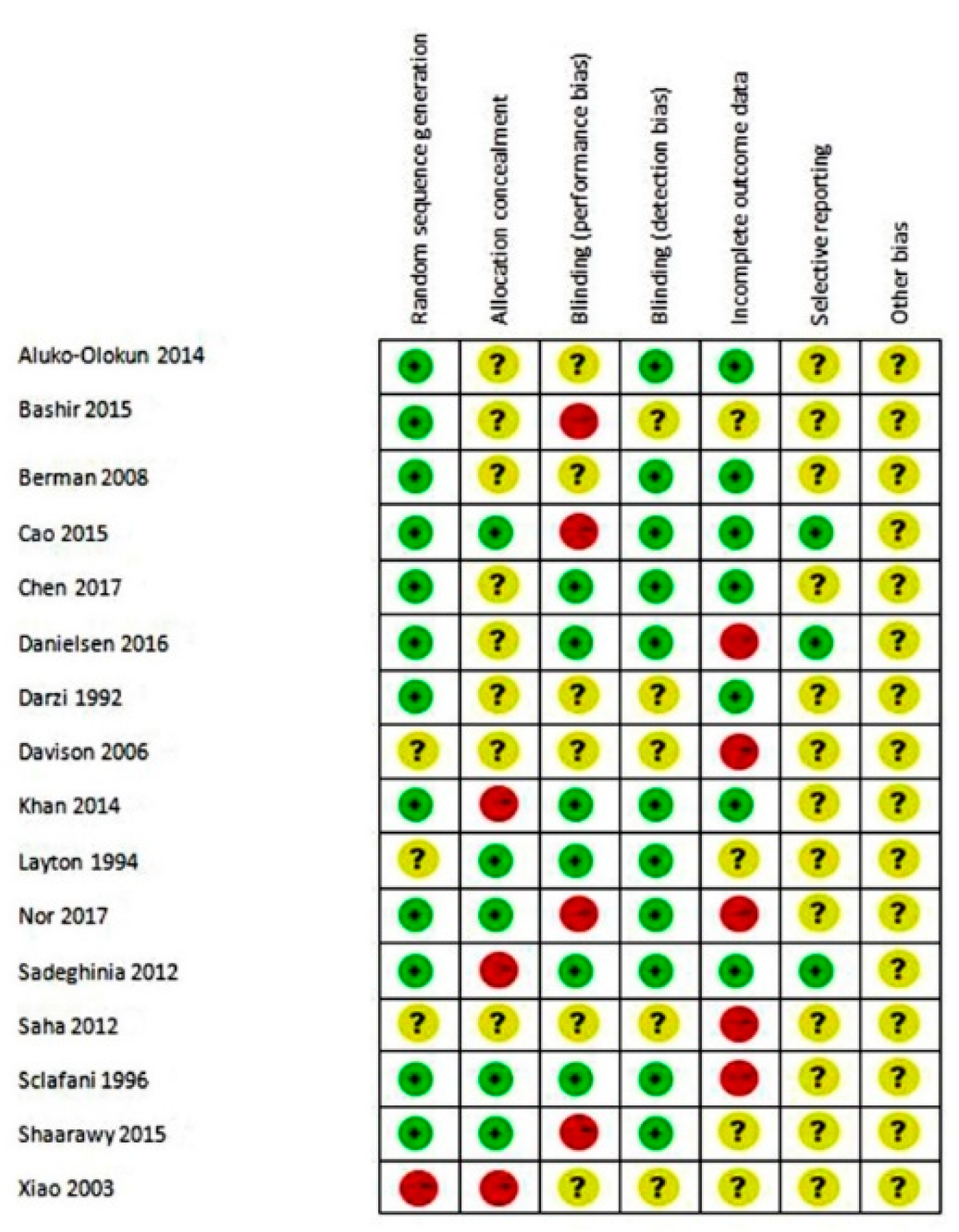
2.3. Meta-Analysis
3. Results
| Study | Comparator | Patients | Site of Lesion | GRADE 1 | |
|---|---|---|---|---|---|
| n | Average age, years | ||||
| Aluko-Olokun, 2014 [50] | Excision with radiotherapy | 107 | 27 | Ear, chest, forehead | MQ |
| Berman, 2008 [42] | Etanercept | 18 | NS 2 | NS 2 | HQ |
| Chen, 2017 [47] | Diprospan + 5-FU, Diprospan + 5-FU + Nd:YAG laser | 69 3 | 27 | Face, neck, trunk, extremities | HQ |
| Darzi, 1992 [51] | Radiotherapy | 65 4 | NS 2 | NS 2 | MQ |
| Khan, 2014 [46] | TAC + 5-FU | 58 | NS 2 | NS 2 | MQ |
| Layton, 1994 [43] | Cryosurgery | 11 | 20 (males); 28 (females) | Acne | HQ |
| Nor, 2017 [52] | Topical clobetasol propionate | 21 5 | 29 | Acne, surgery | MQ |
| Sadeghinia, 2012 [41] | 5-FU | 40 | 43 | Face, neck, trunk, limbs | HQ |
| Saha, 2012 [48] | 5-FU | 50 6 | 33 (TAC); 35 (5-FU) | Operations, acne, burns | MQ |
| Shaarawy, 2015 [53] | Botulinum toxin type A | 24 | 29 | Posttraumatic, idiopathic | HQ |
| Xiao, 2003 [54] | TAC + 5-FU | 214 | 0.3–68 (range) | Chest, shoulder, back | MQ |
| Study | Comparator | Patients | Site of Lesion | GRADE 1 | |
|---|---|---|---|---|---|
| n | Average age, years | ||||
| Bashir, 2015 [55] | 3 TAC injections | 70 | 22 (1 injection); 23 (3 injections) | Ear | HQ |
| Cao, 2015 [45] | TAC + 90Sr-90Y | 61 | 38 | Chest, shoulder, limb, ear, others | HQ |
| Danielsen, 2016 [40] | Verapamil | 14 | 32 | Sternum, shoulder, back, neck, ear, upper arm, forearm, scar area excised | MQ |
| Darzi, 1992 [51] | Radiotherapy | 65 2 | NS 3 | NS 3 | MQ |
| Davison, 2006 [49] | Interferon α-2b | 34 | 30 | Ear, face, abdomen, chest, extremities | MQ |
| Sclafani, 1996 [44] | Radiotherapy | 23 | 27 to 29 | Ear | MQ |
3.1. Studies without Surgery: Primary and Secondary Outcomes
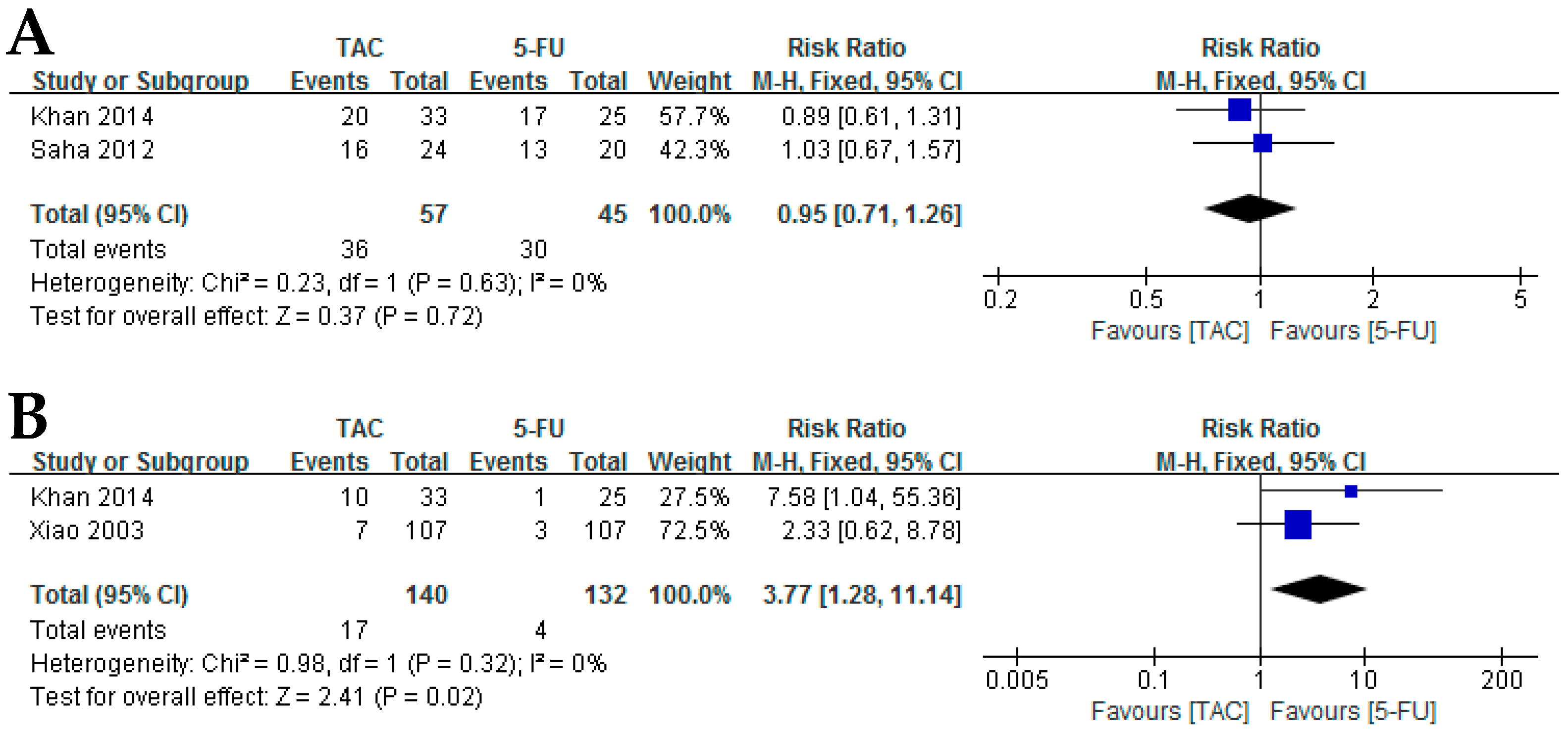
3.1.1. Corticosteroids versus 5-FU
3.1.2. Corticosteroids versus Etanercept
3.1.3. Corticosteroids versus Cryosurgery
3.1.4. Corticosteroids versus Botulinum Toxin Type A
3.1.5. Corticosteroid Injection versus Topical Corticosteroid Cream under Silicone Dressing
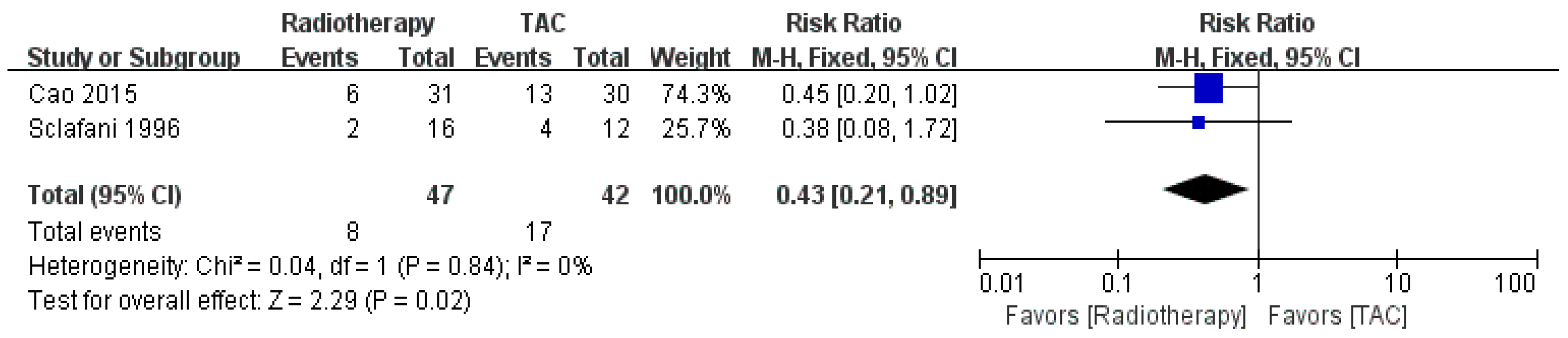
3.1.6. Corticosteroids versus Radiotherapy
3.1.7. Corticosteroids versus Excision Plus Radiotherapy
3.2. Studies with Surgery: Primary and Secondary Outcomes
3.2.1. Single Corticosteroid Injection versus Three Corticosteroid Injections
3.2.2. Preoperative and Postoperative Radiotherapy versus Postoperative Radiotherapy
3.2.3. Corticosteroids versus Radiotherapy
3.2.4. Corticosteroids versus Interferon α-2b
3.2.5. Corticosteroids versus Verapamil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BT | Brachytherapy |
| CI | Confidence interval |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluations |
| FU | Fluorouracil |
| POSAS | Patient and Observer Scar Assessment Scale |
| RCT | Randomised controlled trial |
| RR | Risk ratio |
| TAC | Triamcinolone acetonide |
| VSS | Vancouver Scar Scale |
References
- Peacock, E.E., Jr.; Madden, J.W.; Trier, W.C. Biologic basis for the treatment of keloids and hypertrophic scars. South Med. J. 1970, 63, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.T.; Bhageshpur, R.; DiNick, V.; Khan, A.; Bhaloo, S. Investigation of recurrence rates among earlobe keloids utilizing various postoperative therapeutic modalities. Eur. J. Plast. Surg. 2001, 24, 88–95. [Google Scholar] [CrossRef]
- Seifert, O.; Mrowietz, U. Keloid scarring: Bench and bedside. Arch. Dermatol. Res. 2009, 301, 259–272. [Google Scholar] [CrossRef]
- Shirakami, E.; Yamakawa, S.; Hayashida, K. Strategies to prevent hypertrophic scar formation: A review of therapeutic interventions based on molecular evidence. Burns Trauma 2020, 8, tkz003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, H.P.; Desmouliere, A.; Diegelmann, R.F.; Cohen, I.K.; Compton, C.C.; Garner, W.L.; Kapanci, Y.; Gabbiani, G. Morphological and immunochemical differences between keloid and hypertrophic scar. Am. J. Pathol. 1994, 145, 105–113. [Google Scholar]
- Glass, D.A., 2nd. Current understanding of the genetic causes of keloid formation. J. Investig. Dermatol. Symp. Proc. 2017, 18, S50–S53. [Google Scholar] [CrossRef] [Green Version]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Alhady, S.M.; Sivanantharajah, K. Keloids in various races. A review of 175 cases. Plast. Reconstr. Surg. 1969, 44, 564–566. [Google Scholar] [CrossRef]
- Brissett, A.E.; Sherris, D.A. Scar contractures, hypertrophic scars, and keloids. Facial. Plast Surg. 2001, 17, 263–272. [Google Scholar] [CrossRef]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef]
- Pollack, S.V.; Goslen, J.B. The surgical treatment of keloids. J. Dermatol. Surg. Oncol. 1982, 8, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Cosman, B.; Wolff, M. Correlation of keloid recurrence with completeness of local excision. A negative report. Plast. Reconstr. Surg. 1972, 50, 163–166. [Google Scholar] [CrossRef]
- Siotos, C.; Uzosike, A.C.; Hong, H.; Seal, S.M.; Rosson, G.D.; Cooney, C.M.; Cooney, D.S. Keloid Excision and Adjuvant Treatments: A Network Meta-analysis. Ann. Plast. Surg. 2019, 83, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Li, J.Z.; Chen, S.; Chan, J.Y.; Gao, W. The efficacy of triamcinolone acetonide in keloid treatment: A systematic review and meta-analysis. Front. Med. 2016, 3, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.A.; Davidson, T.M. Scar management: Prevention and treatment strategies. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 242–247. [Google Scholar] [CrossRef]
- Jalali, M.; Bayat, A. Current use of steroids in management of abnormal raised skin scars. Surgeon 2007, 5, 175–180. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, L.; Wang, R.; Cen, Y.; Li, Z. Effects and safety of triamcinolone acetonide-controlled common therapy in keloid treatment: A Bayesian network meta-analysis. Ther. Clin. Risk Manag. 2018, 14, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Chowdri, N.A.; Masarat, M.; Mattoo, A.; Darzi, M.A. Keloids and hypertrophic scars: Results with intraoperative and serial postoperative corticosteroid injection therapy. Aust. N. Z. J. Surg. 1999, 69, 655–659. [Google Scholar] [CrossRef] [Green Version]
- Niessen, F.B.; Spauwen, P.H.; Schalkwijk, J.; Kon, M. On the nature of hypertrophic scars and keloids: A review. Plast. Reconstr. Surg. 1999, 104, 1435–1458. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liu, Y.K.; Qing, C.; Lu, S.L. A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann. Plast. Surg. 2009, 63, 688–692. [Google Scholar]
- Manuskiatti, W.; Fitzpatrick, R.E. Treatment response of keloidal and hypertrophic sternotomy scars: Comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch. Dermatol. 2002, 138, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Alster, T. Laser scar revision: Comparison study of 585-nm pulsed dye laser with and without intralesional corticosteroids. Dermatol. Surg. 2003, 29, 25–29. [Google Scholar] [CrossRef]
- Asilian, A.; Darougheh, A.; Shariati, F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol. Surg. 2006, 32, 907–915. [Google Scholar] [PubMed]
- Finken, M.J.; Mul, D. Cushing’s syndrome and adrenal insufficiency after intradermal triamcinolone acetonide for keloid scars. Eur. J. Pediatr. 2010, 169, 1147–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Leeuwen, M.C.; Stokmans, S.C.; Bulstra, A.E.; Meijer, O.W.; Heymans, M.W.; Ket, J.C.; Ritt, M.J.; van Leeuwen, P.A.; Niessen, F.B. Surgical excision with adjuvant irradiation for treatment of keloid scars: A systematic review. Plast. Reconstr. Surg. Glob. Open 2015, 3, e440. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, N.; Cognetta, A., Jr.; Goldberg, D. Radiation therapy for the adjunctive treatment of surgically excised keloids: A review. J. Clin. Aesthet. Dermatol. 2017, 10, 12–15. [Google Scholar]
- Yamawaki, S.; Naitoh, M.; Ishiko, T.; Muneuchi, G.; Suzuki, S. Keloids can be forced into remission with surgical excision and radiation, followed by adjuvant therapy. Ann. Plast. Surg. 2011, 67, 402–406. [Google Scholar] [CrossRef]
- Recalcati, S.; Caccialanza, M.; Piccinno, R. Postoperative radiotherapy of auricular keloids: A 26-year experience. J. Dermatolog. Treat. 2011, 22, 38–42. [Google Scholar] [CrossRef]
- Bennett, K.G.; Kung, T.A.; Hayman, J.A.; Brown, D.L. Treatment of keloids with excision and adjuvant radiation: A single center experience and review of the literature. Ann. Plast. Surg. 2017, 78, 157–161. [Google Scholar] [CrossRef]
- Ogawa, R.; Yoshitatsu, S.; Yoshida, K.; Miyashita, T. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast. Reconstr. Surg. 2009, 124, 1196–1201. [Google Scholar] [CrossRef]
- Jones, K.; Fuller, C.D.; Luh, J.Y.; Childs, C.C.; Miller, A.R.; Tolcher, A.W.; Herman, T.S.; Thomas, C.R., Jr. Case report and summary of literature: Giant perineal keloids treated with post-excisional radiotherapy. BMC Dermatol. 2006, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speranza, G.; Sultanem, K.; Muanza, T. Descriptive study of patients receiving excision and radiotherapy for keloids. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Borok, T.L.; Bray, M.; Sinclair, I.; Plafker, J.; LaBirth, L.; Rollins, C. Role of ionizing irradiation for 393 keloids. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 865–870. [Google Scholar] [CrossRef]
- Mankowski, P.; Kanevsky, J.; Tomlinson, J.; Dyachenko, A.; Luc, M. Optimizing radiotherapy for keloids: A meta-analysis systematic review comparing recurrence rates between different radiation modalities. Ann. Plast. Surg. 2017, 78, 403–411. [Google Scholar] [CrossRef]
- Nie, Z.; Bayat, A.; Behzad, F.; Rhodes, L.E. Positive response of a recurrent keloid scar to topical methyl aminolevulinate-photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2010, 26, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Biswas, A.; Subramanian, A.; Srinivasan, A.; Choolani, M.; Bongso, A. Human keloid cell characterization and inhibition of growth with human Wharton’s jelly stem cell extracts. J. Cell Biochem. 2014, 115, 826–838. [Google Scholar] [CrossRef]
- Baryza, M.J.; Baryza, G.A. The Vancouver Scar Scale: An administration tool and its interrater reliability. J. Burn. Care Rehabil. 1995, 16, 535–538. [Google Scholar] [CrossRef]
- Draaijers, L.J.; Tempelman, F.R.; Botman, Y.A.; Tuinebreijer, W.E.; Middelkoop, E.; Kreis, R.W.; van Zuijlen, P.P. The patient and observer scar assessment scale: A reliable and feasible tool for scar evaluation. Plast. Reconstr. Surg. 2004, 113, 1960–1965. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: Oxford, UK, 2011. [Google Scholar]
- Danielsen, P.L.; Rea, S.M.; Wood, F.M.; Fear, M.W.; Viola, H.M.; Hool, L.C.; Gankande, T.U.; Alghamdi, M.; Stevenson, A.W.; Manzur, M.; et al. Verapamil is less effective than triamcinolone for prevention of keloid scar recurrence after excision in a randomized controlled trial. Acta Derm. Venereol. 2016, 96, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Sadeghinia, A.; Sadeghinia, S. Comparison of the efficacy of intralesional triamcinolone acetonide and 5-fluorouracil tattooing for the treatment of keloids. Dermatol. Surg. 2012, 38, 104–109. [Google Scholar] [CrossRef]
- Berman, B.; Patel, J.K.; Perez, O.A.; Viera, M.H.; Amini, S.; Block, S.; Zell, D.; Tadicherla, S.; Villa, A.; Ramirez, C.; et al. Evaluating the tolerability and efficacy of etanercept compared to triamcinolone acetonide for the intralesional treatment of keloids. J. Drugs Dermatol. 2008, 7, 757–761. [Google Scholar]
- Layton, A.M.; Yip, J.; Cunliffe, W.J. A comparison of intralesional triamcinolone and cryosurgery in the treatment of acne keloids. Br. J. Dermatol. 1994, 130, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.P.; Gordon, L.; Chadha, M.; Romo, T., 3rd. Prevention of earlobe keloid recurrence with postoperative corticosteroid injections versus radiation therapy: A randomized, prospective study and review of the literature. Dermatol. Surg. 1996, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y.; Shuai, M.S.; Ding, Z.Q.; Chen, S.T.; Zhao, J.L. Clinical observation of the therapeutic efficacy of surgical excision combined with triamcinolone acetonide injection and 90Sr-90Y applicator brachytherapy for the treatment of keloids. J. Clin. Dermatol. 2015, 44, 813–815. [Google Scholar]
- Khan, M.A.; Bashir, M.M.; Khan, F.A. Intralesional triamcinolone alone and in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. J. Pak. Med. Assoc. 2014, 64, 1003–1007. [Google Scholar]
- Chen, X.E.; Liu, J.; Bin Jameel, A.A.; Valeska, M.; Zhang, J.A.; Xu, Y.; Liu, X.W.; Zhou, H.; Luo, D.; Zhou, B.R. Combined effects of long-pulsed neodymium-yttrium-aluminum-garnet laser, diprospan and 5-fluorouracil in the treatment of keloid scars. Exp. Ther. Med. 2017, 13, 3607–3612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, A.K.; Mukhopadhyay, M. A comparative clinical study on role of 5-flurouracil versus triamcinolone in the treatment of keloids. Indian J. Surg. 2012, 74, 326–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, S.P.; Mess, S.; Kauffman, L.C.; Al-Attar, A. Ineffective treatment of keloids with interferon alpha-2b. Plast Reconstr Surg 2006, 117, 247–252. [Google Scholar] [CrossRef]
- Aluko-Olokun, B.; Olaitan, A.A.; Ladeinde, A.L.; Oginni, F.O. The facial keloid: A comparison of treatment outcome between intralesional steroid injection and excision combined with radiotherapy. Eur. J. Plast. Surg. 2014, 37, 361–366. [Google Scholar] [CrossRef]
- Darzi, M.A.; Chowdri, N.A.; Kaul, S.K.; Khan, M. Evaluation of various methods of treating keloids and hypertrophic scars: A 10-year follow-up study. Br. J. Plast Surg. 1992, 45, 374–379. [Google Scholar] [CrossRef]
- Nor, N.M.; Ismail, R.; Jamil, A.; Shah, S.A.; Imran, F.H. A randomized, single-blind trial of clobetasol propionate 0.05% cream under silicone dressing occlusion versus intra-lesional triamcinolone for treatment of keloid. Clin. Drug Investig. 2017, 37, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, E.; Hegazy, R.A.; Abdel Hay, R.M. Intralesional botulinum toxin type A equally effective and better tolerated than intralesional steroid in the treatment of keloids: A randomized controlled trial. J. Cosmet. Dermatol. 2015, 14, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Shu, Y.; Xie, H.; Li, J.; Liu, Z. The observation on the effect of local injection with triamicnolon acetonide acetate and flurouracil on treating keloid. Chin. J. Derm. 2003, 17, 323–324. [Google Scholar]
- Bashir, M.M.; Ahmad, H.; Yousaf, N.; Khan, F.A. Comparison of single intra operative versus an intra operative and two post operative injections of the triamcinolone after wedge excision of keloids of helix. J. Pak. Med. Assoc. 2015, 65, 737–741. [Google Scholar]
- Bloemen, M.C.; van der Veer, W.M.; Ulrich, M.M.; van Zuijlen, P.P.; Niessen, F.B.; Middelkoop, E. Prevention and curative management of hypertrophic scar formation. Burns 2009, 35, 463–475. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, X.; Wei, Z.; Lin, W.; Fan, B.; Feng, S. Efficacy and safety of triamcinolone acetonide alone and in combination with 5-fluorouracil for treating hypertrophic scars and keloids: A systematic review and meta-analysis. Int. Wound J. 2017, 14, 480–487. [Google Scholar] [CrossRef]
- Bi, M.; Sun, P.; Li, D.; Dong, Z.; Chen, Z. Intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid: A systematic review and meta-analysis. Med. Sci. Monit. 2019, 25, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Abedini, R.; Sasani, P.; Mahmoudi, H.R.; Nasimi, M.; Teymourpour, A.; Shadlou, Z. Comparison of intralesional verapamil versus intralesional corticosteroids in treatment of keloids and hypertrophic scars: A randomized controlled trial. Burns 2018, 44, 1482–1488. [Google Scholar] [CrossRef]
- Guix, B.; Henríquez, I.; Andrés, A.; Finestres, F.; Tello, J.I.; Martínez, A. Treatment of keloids by high-dose-rate brachytherapy: A seven-year study. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 167–172. [Google Scholar] [CrossRef]
- Shin, J.Y.; Lee, J.W.; Roh, S.G.; Lee, N.H.; Yang, K.M. A comparison of the effectiveness of triamcinolone and radiation therapy for ear keloids after surgical excision: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2016, 137, 1718–1725. [Google Scholar] [CrossRef]
- Berman, B.; Flores, F. Recurrence rates of excised keloids treated with postoperative triamcinolone acetonide injections or interferon alfa-2b injections. J. Am. Acad. Dermatol. 1997, 37, 755–757. [Google Scholar] [CrossRef]
- Klotz, T.; Munn, Z.; Aromataris, E.C.; Greenwood, J.E. Imiquimod to prevent keloid recurrence postexcision: A systematic review and meta-analysis. Wound Repair Regen. 2020, 28, 145–156. [Google Scholar] [CrossRef] [PubMed]
| Number | Search Strategy |
|---|---|
| 1 | MeSH descriptor: [keloid] explode all trees |
| 2 | MeSH descriptor: [cicatrix, hypertrophic] explode all trees |
| 3 | (Keloid* or hypertrophic or hypertrophic or cicatrix): ti, ab, kw |
| 4 | (“Scar” or “scars” or scarred or scarring): ti, ab, kw |
| 5 | 1 OR 2 OR 3 OR 4 |
| 6 | MeSH descriptor: [radiotherapy] explode all trees |
| 7 | (Radiotherapy* or “radiation therapy”): ti, ab, kw |
| 8 | MeSH descriptor: [adrenal cortex hormones] explode all trees |
| 9 | MeSH descriptor: [steroids] explode all trees |
| 10 | (Corticosteroid* or “hydrocortisone acetate” or methylprednisolone or dexamethasone or triamcinolone or steroid* or betamethasone or glucocort*): ti, ab, kw |
| 11 | 6 OR 7 OR 8 OR 9 OR 10 |
| 12 | 5 AND 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Danielsen, P.L.; Ågren, M.S.; Duke, J.; Wood, F.; Zeng, X.-X.; Mao, Y.; Cen, Y. Corticosteroid Injection Alone or Combined with Surgical Excision of Keloids versus Other Therapies Including Ionising Radiotherapy: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Eur. Burn J. 2021, 2, 41-54. https://0-doi-org.brum.beds.ac.uk/10.3390/ebj2020004
Wang R, Danielsen PL, Ågren MS, Duke J, Wood F, Zeng X-X, Mao Y, Cen Y. Corticosteroid Injection Alone or Combined with Surgical Excision of Keloids versus Other Therapies Including Ionising Radiotherapy: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. European Burn Journal. 2021; 2(2):41-54. https://0-doi-org.brum.beds.ac.uk/10.3390/ebj2020004
Chicago/Turabian StyleWang, Ru, Patricia L. Danielsen, Magnus S. Ågren, Janine Duke, Fiona Wood, Xiao-Xi Zeng, Yu Mao, and Ying Cen. 2021. "Corticosteroid Injection Alone or Combined with Surgical Excision of Keloids versus Other Therapies Including Ionising Radiotherapy: A Systematic Review and Meta-Analysis of Randomised Controlled Trials" European Burn Journal 2, no. 2: 41-54. https://0-doi-org.brum.beds.ac.uk/10.3390/ebj2020004







