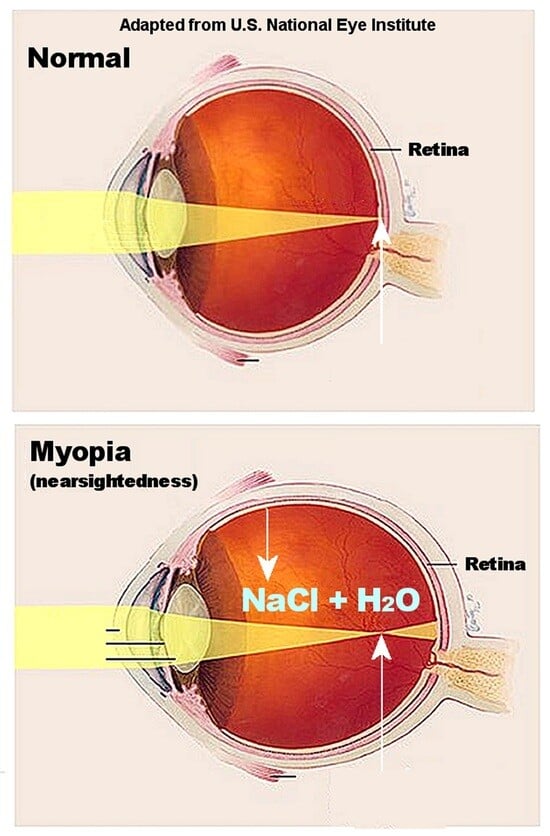
Myopia, Sodium Chloride, and Vitreous Fluid Imbalance: A Nutritional Epidemiology Perspective
Abstract
:1. Introduction
2. Myopia History and Pathophysiology
3. Nutritional Epidemiology of Myopia and Dietary Sodium
4. Sodium Chloride, Osmolality, and Myopia
5. Vitreous Fluid Accumulation
6. Future Myopia Research
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorn, F.; Cruz, A.A.; Machado, A.J.; Carvalho, R.A. Refractive status of indigenous people in the northwestern Amazon region of Brazil. Optom. Vis. Sci. 2005, 82, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, Y.; Chen, L.; Dong, M.; Tang, W.; Chen, S.; Qu, J.; Zhou, X.; Zhi, Z. Near work induces myopia in Guinea pigs. Exp. Eye Res. 2022, 224, 109202. [Google Scholar] [CrossRef] [PubMed]
- Pärssinen, O.; Kauppinen, M. Associations of near work time, watching TV, outdoors time, and parents’ myopia with myopia among school children based on 38-year-old historical data. Acta Ophthalmol. 2022, 100, e430–e438. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.J.; Cohen, E.L.; Neel, J.V. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation 1975, 52, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Heinbecker, P. Not by bread alone. By Vilhjalmur Stefansson. The MacMillan Company, New York, xvi + 339 pp., 1946. ($3.50). Am. J. Phys. Anthropol. 1947, 5, 104–106. [Google Scholar] [CrossRef]
- Young, F.A.; Leary, G.A.; Baldwin, W.R.; West, D.C.; Box, R.A.; Harris, E.; Johnson, C. The transmission of refractive errors within eskimo families. Am. J. Optom. Arch. Am. Acad. Optom. 1969, 46, 676–685. [Google Scholar] [CrossRef]
- Lougheed, T. The changing landscape of arctic traditional food. Environ. Health Perspect. 2010, 118, A386–A393. [Google Scholar] [CrossRef]
- Sabri, K.; Jindani, Y.; De Melo, M.; Innes, E.; Kioke, S. Current Visual Acuity and Refractive Errors among Indigenous Children in Northern Canada. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3368-A0155. [Google Scholar]
- Kempner, W. Treatment of hypertensive vascular disease with rice diet. Am. J. Med. 1948, 4, 545–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, Y.T.; Huang, W.B.; Liu, R.J.; Zuo, Y.J.; He, L.W.; Zhong, L.T.; Zhang, S.C. Prevalence and characteristics of peripheral myopic retinopathy in Guangzhou office workers. Int. J. Ophthalmol. 2018, 11, 1390–1395. [Google Scholar] [CrossRef]
- Stocker, F.W.; Holt, L.B.; Clower, J.W. Clinical experiments with new ways of influencing intraocular tension; effect of rice diet t. Arch. Ophthalmol. 1948, 40, 46–55. [Google Scholar]
- Harb, E.N.; Wildsoet, C.F. Nutritional Factors and Myopia: An Analysis of National Health and Nutrition Examination Survey Data. Optom. Vis. Sci. 2021, 98, 458–468. [Google Scholar] [CrossRef]
- Shaldon, S. Dietary salt restriction and drug-free treatment of hypertension in ESRD patients: A largely abandoned therapy. Nephrol. Dial. Transpl. 2002, 17, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Qin, Y.; Li, J.; Luo, Z.; Wang, X. Dietary composition plays a crucial role in the development of myopia through the inflammatory pathway: A Mendelian randomization study. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Chua, S.Y.; Sabanayagam, C.; Tan, C.S.; Lim, L.S.; Toh, J.Y.; Chong, Y.S.; Gluckman, P.D.; Yap, F.; Cheng, C.Y.; Ngo, C.S.; et al. Diet and risk of myopia in three-year-old Singapore children: The GUSTO cohort. Clin. Exp. Optom. 2018, 101, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tan, C.S.; Foo, L.L.; Sugianto, R.; Toh, J.Y.; Sun, C.H.; Yap, F.; Sabanayagam, C.; Chong, F.M.; Saw, S.M. Dietary intake and associations with myopia in Singapore children. Ophthalmic Physiol. Opt. 2022, 42, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Chamarty, S.; Gupta, S.K.; Dhakal, R.; Verkicharla, P.K. Is There Any Association between Nutrition and Myopia? A Systematic Review. Optom. Vis. Sci. 2023, 100, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Wolfswinkel, J.F.; Furtmueller, E.; Wilderom, C.P.M. Using grounded theory as a method for rigorously reviewing literature. Eur. J. Inf. Syst. 2013, 22, 45–55. [Google Scholar] [CrossRef]
- Medow, N. Ancient Egyptian Records Provide Clues to Ophthalmic Care. Ophthalmology Times. Available online: https://www.ophthalmologytimes.com/view/ancient-egyptian-records-provide-clues-ophthalmic-care (accessed on 5 October 2023).
- Ikram, S. Meat Preservation in Ancient Egypt. In Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures; Selin, H., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1442–1443. [Google Scholar]
- Trentin, L. Exploring Visual Impairment in Ancient Rome; Brill: Leiden, The Netherlands, 2013. [Google Scholar]
- De Jong, P. Myopia: Its historical contexts. Br. J. Ophthalmol. 2018, 102, 1021–1027. [Google Scholar] [CrossRef]
- Durack, E.; Alonso-Gomez, M.; Wilkinson, M.G. Salt: A review of its role in food science and public health. Curr. Nutr. Food Sci. 2008, 4, 290–297. [Google Scholar] [CrossRef]
- Retief, F.; Stulting, A.; Cilliers, L. The eye in antiquity. S. Afr. Med. J. 2008, 98, 697–700. [Google Scholar]
- Salmon, W. The Works of Aristotle the Famous Philosopher; Good Press, Overdrive: Cleveland, OH, USA, 2023. [Google Scholar]
- Subudhi, P.; Agarwal, P. Myopia. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK580529/ (accessed on 3 September 2023).
- Chua, S.Y.L.; Foster, P.J. The Economic and Societal Impact of Myopia and High Myopia. In Updates on Myopia: A Clinical Perspective; Ang, M., Wong, T.Y., Eds.; Springer: Singapore, 2020; pp. 53–63. [Google Scholar]
- Ueta, T.; Makino, S.; Yamamoto, Y.; Fukushima, H.; Yashiro, S.; Nagahara, M. Pathologic myopia: An overview of the current understanding and interventions. Glob. Health Med. 2020, 2, 151–155. [Google Scholar] [CrossRef]
- National Eye Institute. Myopia and Eye Development. Available online: https://www.youtube.com/watch?v=ttz6FIGhdY0 (accessed on 18 September 2023).
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.S.; Fricke, T.R.; Frick, K.D.; Jong, M.; Naduvilath, T.J.; Resnikoff, S.; Sankaridurg, P. Potential Lost Productivity Resulting from the Global Burden of Myopia: Systematic Review, Meta-analysis, and Modeling. Ophthalmology 2019, 126, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Vilela, M.A.P.; Pellanda, L.C.; Fassa, A.G.; Castagno, V.D. Prevalence of asthenopia in children: A systematic review with meta-analysis. J. Pediatr. 2015, 91, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Lu, S.Y.; Zhang, X.J.; Chen, L.J.; Pang, C.P.; Yam, J.C. Myopia Genetics and Heredity. Children 2022, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Nitzan, I.; Megreli, J.; Derazne, E.; Tzur, D.; Pinhas-Hamiel, O.; Afek, A.; Twig, G. Myopia and BMI: A nationwide study of 1.3 million adolescents. Obesity 2022, 30, 1691–1698. [Google Scholar] [CrossRef]
- Peled, A.; Afek, A.; Twig, G.; Pras, E.; Rotenstreich, Y.; Sher, I.; Derazne, E.; Tzur, D.; Gordon, B. Myopia and Childhood Migration: A Study of 607 862 Adolescents. Ophthalmology 2020, 127, 713–723. [Google Scholar] [CrossRef]
- Ye, Y. A Summary of the Effect of Physical Exercise on Myopia in Children and Adolescents. Open Access Libr. J. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.H.; Lee, D.C. Factors associated with myopia in 19-year-old adult men in Korea between 2014 and 2020. Sci. Rep. 2023, 13, 11581. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Sperduto, R.D.; Ferris, F.L., III. Increased Prevalence of Myopia in the United States Between 1971–1972 and 1999–2004. Arch. Ophthalmol. 2009, 127, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- New York State Department of Health. The Potential Health Impact of Reducing Excess Sodium Consumption. Available online: https://www.health.ny.gov/statistics/brfss/reports/docs/1308_brfss_reduce_sodium.pdf (accessed on 10 August 2023).
- Moubarac, J.-C.; Batal, M.; Martins, A.P.B.; Claro, R.; Levy, R.; Cannon, G.; Monteiro, C. Time trends changes in the consumption of ultra-processed products during the 20th century in Canada. Can. J. Diabetes 2013, 37, S245. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.J.; Lee, K.G.; Kim, J. Obesity and high myopia in children and adolescents: Korea National Health and Nutrition Examination Survey. PLoS ONE 2022, 17, e0265317. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Salt Intake. Pan American Health Organization. Available online: https://www.paho.org/en/enlace/salt-intake (accessed on 15 August 2023).
- NHLBI. Implementing Recommendations for Dietary Salt Reduction: Where Are We? Where Are We Going? How Do We Get There? A Summary of an NHLBI Workshop; National Institutes of Health, National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1996. [Google Scholar]
- Campbell, N.R.; Correa-Rotter, R.; Cappuccio, F.P.; Webster, J.; Lackland, D.T.; Neal, B.; MacGregor, G.A. Proposed nomenclature for salt intake and for reductions in dietary salt. J. Clin. Hypertens. 2015, 17, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Kritika; Sharma, N.; Alam, M.A.; Khan, F.A.; Dhoundiyal, S. Approaching headaches and facial pains in eye care practice. Int. Ophthalmol. 2023, 43, 3433–3444. [Google Scholar] [CrossRef] [PubMed]
- Harle, D.E.; Evans, B.J. The correlation between migraine headache and refractive errors. Optom. Vis. Sci. 2006, 83, 82–87. [Google Scholar] [CrossRef]
- Brown, R.B. Sodium Chloride, Migraine and Salt Withdrawal: Controversy and Insights. Med. Sci. 2021, 9, 67. [Google Scholar] [CrossRef]
- Ghaffari, H.; Grant, S.C.; Petzold, L.R.; Harrington, M.G. Regulation of CSF and brain tissue sodium levels by the blood-CSF and blood-brain barriers during migraine. Front. Comput. Neurosci. 2020, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Woodward, M.; Appel, L.J. Effects of dietary sodium and the DASH diet on the occurrence of headaches: Results from randomised multicentre DASH-Sodium clinical trial. BMJ Open 2014, 4, e006671. [Google Scholar] [CrossRef]
- Mirzababaei, A.; Khorsha, F.; Togha, M.; Yekaninejad, M.S.; Okhovat, A.A.; Mirzaei, K. Associations between adherence to dietary approaches to stop hypertension (DASH) diet and migraine headache severity and duration among women. Nutr. Neurosci. 2020, 23, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Inglese, M.; Madelin, G.; Oesingmann, N.; Babb, J.S.; Wu, W.; Stoeckel, B.; Herbert, J.; Johnson, G. Brain tissue sodium concentration in multiple sclerosis: A sodium imaging study at 3 tesla. Brain 2010, 133, 847–857. [Google Scholar] [CrossRef]
- Zaaraoui, W.; Konstandin, S.; Audoin, B.; Nagel, A.M.; Rico, A.; Malikova, I.; Soulier, E.; Viout, P.; Confort-Gouny, S.; Cozzone, P.J.; et al. Distribution of Brain Sodium Accumulation Correlates with Disability in Multiple Sclerosis: A Cross-sectional 23Na MR Imaging Study. Radiology 2012, 264, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Van der Feen, F.E.; de Haan, G.A.; van der Lijn, I.; Huizinga, F.; Meilof, J.F.; Heersema, D.J.; Heutink, J. Recognizing visual complaints in people with multiple sclerosis: Prevalence, nature and associations with key characteristics of MS. Mult. Scler. Relat. Disord. 2022, 57, 103429. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Nichols, E.; Steiner, T.J.; Abd-Allah, F.; Abdelalim, A.; Al-Raddadi, R.M.; Ansha, M.G.; Barac, A.; Bensenor, I.M.; Doan, L.P. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.-Y.; Gritter, M.; Vogt, L.; de Borst, M.H.; Rotmans, J.I.; Hoorn, E.J. Dietary potassium and the kidney: Lifesaving physiology. Clin. Kidney J. 2020, 13, 952–968. [Google Scholar] [CrossRef]
- Krishna, G.G.; Chusid, P.; Hoeldtke, R.D. Mild potassium depletion provokes renal sodium retention. J. Lab. Clin. Med. 1987, 109, 724–730. [Google Scholar]
- Valero-Morales, I.; Tan, M.; Pei, Y.; He, F.J.; MacGregor, G.A. 24-hour sodium and potassium excretion in the Americas: A systematic review and meta-analysis. Rev. Panam. Salud Publica 2022, 46, e199. [Google Scholar] [CrossRef]
- Strange, K. Cellular volume homeostasis. Adv. Physiol. Educ. 2004, 28, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Harju, M.; Kivelä, T.; Lindbohm, N.; Koivusalo, R.; Paloheimo, M. Intravenous hypertonic saline to reduce intraocular pressure. Acta Ophthalmol. 2013, 91, 625–629. [Google Scholar] [CrossRef]
- Bringmann, A.; Hollborn, M.; Kohen, L.; Wiedemann, P. Intake of dietary salt and drinking water: Implications for the development of age-related macular degeneration. Mol. Vis. 2016, 22, 1437–1454. [Google Scholar]
- Hombrebueno, J.R.; Luo, C.; Guo, L.; Chen, M.; Xu, H. Intravitreal Injection of Normal Saline Induces Retinal Degeneration in the C57BL/6J Mouse. Transl. Vis. Sci. Technol. 2014, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Wei, C.C.; Chang, C.Y.; Chen, T.H.; Hsu, Y.A.; Hsieh, Y.C.; Chen, H.J.; Wan, L. Role of Chronic Inflammation in Myopia Progression: Clinical Evidence and Experimental Validation. EBioMedicine 2016, 10, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Herbort, C.P.; Papadia, M.; Neri, P. Myopia and inflammation. J. Ophthalmic Vis. Res. 2011, 6, 270–283. [Google Scholar] [PubMed]
- Zhang, D.; Wang, C.; Cao, S.; Ye, Z.; Deng, B.; Kijlstra, A.; Yang, P. High-Salt Enhances the Inflammatory Response by Retina Pigment Epithelium Cells following Lipopolysaccharide Stimulation. Mediat. Inflamm. 2015, 2015, 197521. [Google Scholar] [CrossRef]
- Willermain, F.; Libert, S.; Motulsky, E.; Salik, D.; Caspers, L.; Perret, J.; Delporte, C. Origins and consequences of hyperosmolar stress in retinal pigmented epithelial cells. Front. Physiol. 2014, 5, 199. [Google Scholar] [CrossRef]
- Cases, O.; Obry, A.; Ben-Yacoub, S.; Augustin, S.; Joseph, A.; Toutirais, G.; Simonutti, M.; Christ, A.; Cosette, P.; Kozyraki, R. Impaired vitreous composition and retinal pigment epithelium function in the FoxG1::LRP2 myopic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1242–1254. [Google Scholar] [CrossRef]
- Seko, Y.; Shimokawa, H.; Pang, J.; Tokoro, T. Disturbance of electrolyte balance in vitreous of chicks with form-deprivation myopia. Jpn. J. Ophthalmol. 2000, 44, 15–19. [Google Scholar] [CrossRef]
- Pickett-Seltner, R.L.; Doughty, M.J.; Pasternak, J.J.; Sivak, J.G. Proteins of the vitreous humor during experimentally induced myopia. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3424–3429. [Google Scholar]
- Marshall, A.T.; Crewther, S.G. An X-ray microanalytical method for measuring in vivo element and water concentrations, relating to osmoregulation, in cells and tissues of the posterior eye. J. Microsc. 2021, 283, 21–28. [Google Scholar] [CrossRef]
- Törnquist, P.; Alm, A.; Bill, A. Permeability of ocular vessels and transport across the blood-retinal-barrier. Eye 1990, 4, 303–309. [Google Scholar] [CrossRef]
- Bleeker, G.M.; van Haeringen, N.; Glasius, E. Osmotically Induced Change in the Volume of the Vitreous Body Causing Protrusion of the Ocular Diaphragm. Ophthalmologica 2010, 144, 263. [Google Scholar] [CrossRef]
- Kinsey, V.E. Ion movement in the eye. Circulation 1960, 21, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Horng, C.T.; Lee, Y.L.; Wu, H.C.; Lai, H.Y.; Hsu, J.Y.; Hsu, C.W.; Chien, K.J.; Kuo, W.H. High salt diet induced the rapid myopic shift of cataract formation. Life Sci. J. 2014, 11, 396–399. [Google Scholar]
- Zilg, B.; Alkass, K.; Berg, S.; Druid, H. Interpretation of postmortem vitreous concentrations of sodium and chloride. Forensic Sci. Int. 2016, 263, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M. Transient Choroidal Thickening Associated with Hyponatremia. J. Retin. 2018, 3, 92–96. [Google Scholar] [CrossRef]
- Fortune, B.E.; Garcia-Tsao, G. Hypervolemic hyponatremia: Clinical significance and management. Clin. Liver Dis. 2013, 2, 109. [Google Scholar] [CrossRef]
- Muhiddin, H.S.; Mayasari, A.R.; Umar, B.T.; Sirajuddin, J.; Patellongi, I.; Islam, I.C.; Ichsan, A.M. Choroidal Thickness in Correlation with Axial Length and Myopia Degree. Vision 2022, 6, 16. [Google Scholar] [CrossRef]
- Teberik, K.; Kaya, M. Retinal and Choroidal Thickness in Patients with High Myopia without Maculopathy. Pak. J. Med. Sci. 2017, 33, 1438–1443. [Google Scholar] [CrossRef]
- Metlapally, R.; Wildsoet, C.F. Scleral Mechanisms Underlying Ocular Growth and Myopia. Prog. Mol. Biol. Transl. Sci. 2015, 134, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Chen, Y.J.; Chen, C.H.; Chen, Y.H.; Shin, S.J.; Yang, H.J.; Kuo, H.K. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye 2008, 22, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, D.; Prajapati, V.; Patil, M.S.; Singhal, D. A Study on the Association Between Myopia and Elevated Intraocular Pressure Conducted at a Tertiary Care Teaching Hospital in Gujarat, India. Cureus 2022, 14, e28128. [Google Scholar] [CrossRef] [PubMed]
- Abcouwer, S.F.; Shanmugam, S.; Muthusamy, A.; Lin, C.M.; Kong, D.; Hager, H.; Liu, X.; Antonetti, D.A. Inflammatory resolution and vascular barrier restoration after retinal ischemia reperfusion injury. J. Neuroinflamm. 2021, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fang, W.; Hu, F.; Zhou, X.; Cheng, Y.; Jiang, C. A high-salt diet aggravates retinal ischaemia/reperfusion injury. Exp. Eye Res. 2019, 188, 107784. [Google Scholar] [CrossRef]
- Schulz, A.; Wahl, S.; Rickmann, A.; Ludwig, J.; Stanzel, B.V.; von Briesen, H.; Szurman, P. Age-Related Loss of Human Vitreal Viscoelasticity. Transl. Vis. Sci. Technol. 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: The retina and beyond. Acta Neuropathol. 2016, 132, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Razaghi, R.; Padilla, S.; Rahmati, S.M.; Downs, J.C.; Acott, T.S.; Kelley, M.J.; Wang, R.K.; Johnstone, M. Viscoelastic Biomechanical Properties of the Conventional Aqueous Outflow Pathway Tissues in Healthy and Glaucoma Human Eyes. J. Clin. Med. 2022, 11, 6049. [Google Scholar] [CrossRef]
- Tseng, V.L.; Topouzis, F.; Yu, F.; Keskini, C.; Pappas, T.; Founti, P.; Anastasopoulos, E.; Harris, A.; Wilson, M.R.; Coleman, A.L. Association Between Dietary Salt Intake and Open Angle Glaucoma in the Thessaloniki Eye Study. J. Glaucoma 2022, 31, 494–502. [Google Scholar] [CrossRef]
- Deliyanti, D.; Armani, R.; Casely, D.; Figgett, W.A.; Agrotis, A.; Wilkinson-Berka, J.L. Retinal vasculopathy is reduced by dietary salt restriction: Involvement of Glia, ENaCα, and the renin-angiotensin-aldosterone system. Arter. Thromb. Vasc. Biol. 2014, 34, 2033–2041. [Google Scholar] [CrossRef]
- Sherwin, J.C.; Kokavec, J.; Thornton, S.N. Hydration, fluid regulation and the eye: In health and disease. Clin. Exp. Ophthalmol. 2015, 43, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Baser, G.; Cengiz, H.; Uyar, M.; Seker Un, E. Diurnal Alterations of Refraction, Anterior Segment Biometrics, and Intraocular Pressure in Long-Time Dehydration due to Religious Fasting. Semin. Ophthalmol. 2016, 31, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Troilo, D.; Smith, E.L., III; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.-S.; et al. IMI—Report on Experimental Models of Emmetropization and Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef] [PubMed]
- Schaeffel, F.; Feldkaemper, M. Animal models in myopia research. Clin. Exp. Optom. 2015, 98, 507–517. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.B. Myopia, Sodium Chloride, and Vitreous Fluid Imbalance: A Nutritional Epidemiology Perspective. Epidemiologia 2024, 5, 29-40. https://0-doi-org.brum.beds.ac.uk/10.3390/epidemiologia5010003
Brown RB. Myopia, Sodium Chloride, and Vitreous Fluid Imbalance: A Nutritional Epidemiology Perspective. Epidemiologia. 2024; 5(1):29-40. https://0-doi-org.brum.beds.ac.uk/10.3390/epidemiologia5010003
Chicago/Turabian StyleBrown, Ronald B. 2024. "Myopia, Sodium Chloride, and Vitreous Fluid Imbalance: A Nutritional Epidemiology Perspective" Epidemiologia 5, no. 1: 29-40. https://0-doi-org.brum.beds.ac.uk/10.3390/epidemiologia5010003