Reversible Metal Ion/Complex Binding to Chitin Controlled by Ligand, Redox, and Photochemical Reactions and Active Movement of Chitin on Aquatic Arthropods
Abstract
:1. Introduction
1.1. Introduction
- Hot water, possibly causing the hydrolysis of chitin acetamide groups to yield chitosan or even (N-acetyl-)glucosamine;
- Adsorbed and photoexcited Eu(III);
- Free radicals made from co-adsorbed organic matter attacked by Eu(III)*.
- (1)
- Constructing model devices to study the photochemical effects of chitin’s retention of certain ions, especially Eu, with an emphasis on:
- (2)
- (3)
- Photoredox—(Eu and U, possibly Fe and Cu);
- The cleavage of M-CH3- (pnictogens, Hg, Sn, and Pb);
- M’-CO bonds, with [M(CO)6] and [Fe(CO)5] formed in and vented from landfills [17].
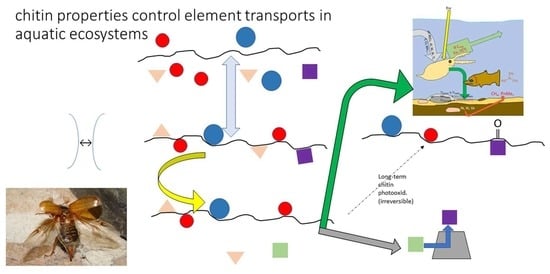
1.2. Hypothesis
- (a)
- Chitin-covered plankton dwells close to the surface during daylight hours;
- (b)
- There is sufficient and appropriate C;Norg in water.
2. Materials and Methods
- Dark, organic matter only;
- The addition of Eu(III);
- The start of illumination, studying events/changes taking place just after light was admitted (within a few s);
- Measuring changes in prolonged irradiation, extending over several days until there was no photoinduced voltage or current left.
3. Results and Discussion
3.1. Results in Lab Systems and Using Isolated Chitin
- (a)
- H2;
- (b)
3.2. Chitin in Organisms, Multilayer Films on Them and Pathways of Metals, Signal Compounds in Eusocial Arthropods: Evidence from Ants
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| β | complex formation constant. Depends on T and solvent for a given complex such as [LaCl]2+ or [Fe(glyc]2+ |
| DMF | solvent N,N-dimethyl formamide. Fully miscible with water and aromatics but not with aliphatic hydrocarbons, perfluorinated organics, or long-chain ethers. Best solvent for performing photoreduction of Eu(III) when combined with organic matter. |
| DOC | Dissolved organic carbon. Usually < 10% of total limnetic C content. Main component is polysaccharide |
| DON | dissolved organic nitrogen. Can be both highly reactive in biological terms (amino acids) or persist over centuries. |
| ε | Redox potential vs. some standard electrode, often → SCE. |
| H0 | Chemical potential of solvated proton in non-aq. media. Concerning fully dissociated acids such as HClO4 or HSO3CF3 in CH3CN or → DMF, H0 = 0 for a 1 M solution. The most acidic solutions which can be prepared (H0 < −20) correspond to almost bare protons, with species such as H2F+, HCO+, H2SO3F+, or even H4O2+, H3+ just weakly interacting with anions such as SbF6− and its SO3 solvates in such (viscous, highly associated) media, such as “magic acid” or HF/SbF5 (the main problems in making such solutions are their corrosive properties and the fact that few strong fluoride acceptors do readily dissolve in either HFliq or HSO3F [79]). In water, H0 = pH. Buffer systems in non-aq. solutions will have a H0 value different from pH in water, e.g., glycine in DMF (11.0 [39] rather than 6.1) |
| HM | heavy metals. Here, denominating metals have an elemental density ρ ≥ 6 g/cm3, thus deliberately excluding elements which differ massively from “typical” HM in their chemical properties while their densities are 5–6 g/cm3, like Eu (unlike all other REEs), Ra, Ga, and V. Some non-metals sometimes are mis-labeled as HM for their toxic properties, especially As, Sb, and Te. |
| LM | Light metals. All metal-forming elements with low(-er) densities (see HM). |
| LREE | Light rare-earth elements. Unprecise definition but often used to summarize elements Z = 57–63 (La–Eu). |
| POC | Particulate organic carbon. In lakes, POC sometimes forms “lake snow”. Consists of suspended biopolymers (chitin, cellulose, and insoluble salts with organic anions) and dead plankton. |
| REE | Rare-earth elements. All the metals with Z = 57–71 (La–Lu), sometimes including yttrium. Highly reactive, electropositive metals (Eu and Yb even dissolve in liquid ammonia [80], like alkali metals and heavy alkaline earths) among which, Eu has unique photochemical properties. Mostly trivalent, while Eu, and to a lesser extent Yb (also in water), Sm, Tm, Nd, and Dy (in solvents such as THF, series of decreasing stability) form M2+ ions and M(IV) can be prepared for Ce (various media), Pr, Nd, Tb, and Dy (in solvents HF, BrF5, or solid state only). Peculiar spectroscopic properties due to partly vacant f orbitals are used in solid-state lasers and fluorescent agents. REE alloys (Nd, Sm, and Dy with Fe or Co) form the most powerful ferromagnetic materials known. |
| σ | L.P. Hammett´s (1893–1987) substituent constant which originally described changes in the reactivity of benzene or benzoic acid towards electrophiles; oxidantsor side chain reactants are observed when the aromatic ring is endowed with some substituent ≠ H, COO−. The Hammett parameter can also be used to define binding in and electrochemical properties of metal complexes, with σ describing binding centers in the ligand [12]. |
| SCE | saturated (6 M KCl solution) calomel electrode; redox couple |
References
- Pinto, P.X.; Al-Abed, S.R.; Reisman, D.J. Biosorption of heavy metals from mining influenced water onto chitin products. Chem. Eng. J. 2011, 166, 1002–1009. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin adsorbents for toxic metals: A review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.A.; Rocchetti, R.; Marangio, G. Separation of zirconium, niobium, cerium and ruthenium on chitin and chitosan columns for the determination of cesium in nuclear fuel solutions. J. Radioanal. Chem. 1972, 10, 17–25. [Google Scholar] [CrossRef]
- Ishay, J.S.; Benshalom-Shimony, T.; Ben-Shalom, A.; Kristianpoller, N. Photovoltaic effects in the Oriental hornet, Vespa orientalis. J. Insect Physiol. 1992, 38, 37–48. [Google Scholar] [CrossRef]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from Parapenaeus longirostris from Moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Agarwal, S.; Mast, C.; Dehnicke, K.; Greiner, A. Rare earth metal initiated ring-opening polymerization of lactones. Macromol. Rapid Commun. 2000, 21, 195–198. [Google Scholar] [CrossRef]
- Bauer, A. Orientierende Untersuchungen zur Bindung von Metallionen an Chitin und zur Davon Abhängigen Eignung von Arthropoden zur Bestimmung von Metallionenkonzentrationen in der Umwelt. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2014. [Google Scholar]
- Vijayaraghavan, K.; Balasubramanian, R. Single and binary biosorption of cerium and europium onto crab shell particles. Chem. Engin. J. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Fränzle, S.; Erler, M.; Blind, F.; Ariuntsetseg, L.; Narangarvuu, D. Chitin adsorption in environmental monitoring: Not an alternative to moss monitoring but a method providing (lots of) bonus information. J. Sci. Arts Univ. 2019, 19, 659–674. [Google Scholar]
- Erler, M. Untersuchung des Bindungsverhaltens Ausgewählter Elemente und Ihrer Bodenrelevanten Komplexe an Chitin. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2020. [Google Scholar]
- Blind, F. Orientierende Untersuchungen zur Platinmetall Freien Aktivierung von CH-Bindungen für Europium Basierte Brennstoffzellenanwendungen. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2018. [Google Scholar]
- Blind, F.; Fränzle, S. Chitin as a sorbent superior to other biopolymers: Features and applications in environmental research, energy conversion, and understanding evolution of animals. Polysaccharides 2021, 2, 773–794. [Google Scholar] [CrossRef]
- Luoga, Y.F. Potential Influence of Exposed Lignite on Local Biota and Biodiversity. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2020. [Google Scholar]
- Nickens, K.P.; Patierno, S.R.; Ceryak, S. Chromium genotoxicity: A double-edged sword. Chem. Biol. Interact. 2010, 188, 276–288. [Google Scholar] [CrossRef] [Green Version]
- Retschke, D. Orientierende Untersuchungen zur Adsorption von Schwermetallen (Nickel) unter dem Einfluss Ausgewählter Komplexliganden Sowie in Arealen Potenzieller und Manifester Methanogenese. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2017. [Google Scholar]
- Schieritz, R. (IHI (International Institute) Zittau, TU Dresden, Zittau, Germany); Fränzle, S. (IHI (International Institute) Zittau, TU Dresden, Zittau, Germany). Investigation into adsorption of neutral biogenic organometal entities (pnictogen trimethyls, Fe, Mo, and W carbonyls) on chitin and chemical (I2, dioxygen complexes) photochemical modification of sorbate films thus produced. unpublished results. 2015. [Google Scholar]
- Feldmann, J.; Cullen, W.R. Occurrence of volatile transition metal compounds in landfill gas: Synthesis of molybdenum and tungsten carbonyls in the environment. Environ. Sci. Technol. 1997, 31, 2125–2129. [Google Scholar] [CrossRef]
- Yang, X.; Yin, D.; Sun, H.; Wang, X.; Dai, L.; Chen, Y.; Cao, M. Distribution and bioavailability of rare earth elements in aquatic microcosm. Chemosphere 1999, 39, 2443–2450. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH diagrams: Intercomparison of thermodynamic databases. Geol. Surv. Jpn. Open File Rep. 2005, 419, 102. [Google Scholar]
- Bolsunovsky, A.; Melgunov, M.; Chuguevskii, A.; Lind, O.C.; Salbu, B. Unique diversity of radioactive particles found in the Yenisei River floodplain. Sci. Rep. 2017, 7, 11132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curragh, E.F.; Henbest, H.B.; Thomas, A. Amine oxidation. Part VII. Cleavage of tetra-N-substituted 1,2-diamines by manganese dioxide. J. Chem. Soc. 1960, 3559–3565. [Google Scholar] [CrossRef]
- Tanner, S.P.; Choppin, G.R. Lanthanide and actinide complexes of glycine. Inorg. Chem. 1968, 7, 1268–1271. [Google Scholar]
- Matsumoto, A.; Azuma, N. Photodecomposition of europium(III) acetate and formate in aqueous solutions. J. Phys. Chem. 1988, 92, 1830–1835. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, Y.; Pang, S.-Y.; Lu, X.-T.; Zhou, Y.; Ma, J.; Wang, Q. Understanding the role of manganese dioxide in the oxidation of phenolic compounds by aqueous permanganate. Env. Sci. Technol. 2015, 49, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Karraker, R.H. Stability Constants of Some Rare-Earth Metal Chelates. Ph.D. Thesis, Iowa State University of Science and Technology, Ames, IA, USA, 1961. [Google Scholar]
- Moeller, T.; Martin, D.F.; Thompson, L.C.; Ferrus, R.; Feistel, G.R.; Randall, W.J. The coordination chemistry of yttrium and the rare earth metal ions. Chem. Rev. 1965, 65, 1–50. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; van Horn, D.J.; Shah, J.J.F.; Findlay, S. Ecoenzymatic stoichiometry in relation to productivity for freshwater biofilm and plankton communities. Microb. Ecol. 2010, 60, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Schwoerbel, J.; Brendelberger, H. Einführung in die Limnologie, 10th ed.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Thompson, A.J.; Sinsabaugh, R.L. Matrix- and particulate phosphatase and aminopeptidase activity in limnetic biofilms. Aquat. Microb. Ecol. 2000, 21, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Loppi, S.; Vannini, A.; Monaci, F.; Dagodzo, D.; Blind, F.; Erler, M.; Fränzle, S. Can chitin and chitosan replace the lichen Evernia prunastri for environmental biomonitoring of Cu and Zn air contamination? Biology 2020, 9, 301. [Google Scholar] [CrossRef]
- Jurkowski, W.; Paper, M.; Brück, T.B. Isolation and Investigation of natural rare earth metal chelating agents from Calothrix brevissima-A Step Towards Unraveling the Mechanisms of Metal Biosorption. Front. Bioeng. Biotechnol. 2022, 10, 833112. [Google Scholar] [CrossRef] [PubMed]
- Budelmann, P. Verbreitung der Flusskrebse (Decapoda) in der Südlichen Oberlausitz und die Eignung des Invasiven Kamberkrebses (Orconectes Limosus) für Chitin-Basiertes Monitoring von Schwermetallen in Limnischen Ökosystemen. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2021. [Google Scholar]
- Brandys, M.; Stein, G. Photochemical evolution of hydrogen from aqueous solutions of europium ions at lambda > 310 nm. The role of europium(II). J. Phys. Chem. 1978, 82, 852–854. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements and yttrium. 2. Theoretical predictions of speciation in hydrothermal solutions to 350 °C at saturation water vapor pressure. Chem. Geol. 1990, 88, 99–125. [Google Scholar] [CrossRef]
- Gebauer, T. Methodische Optimierung des Übertrags von Metallionen aus Umweltprobenmodellen auf Chitinoberflächen und von Diesen zu Zwecken Analytischen Biomonitorings sowie Untersuchungen zur Diffusion/Ausbreitung von Analyten in Chitinproben. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2016. [Google Scholar]
- Pulsfort, J. Biopolymere Als Potenzielle Substrate der Photooxidation Durch f-Block-Metalle. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2021. [Google Scholar]
- Malhotra, N.; Hsu, H.-S.; Liang, S.-T.; Jmelou, M.; Roldan, M.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. An Updated Review of Toxicity Effect of the Rare Earth Elements (REEs) on Aquatic Organisms. Animals 2020, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, S.; Callac, N.; Allard, B.; Smittenberg, R.H.; Dupraz, C. Microbial communities inhabiting a rare earth element enriched birnessite-type manganese deposit in the Ytterby Mine, Sweden. Geomicrobiol. J. 2018, 35, 657–674. [Google Scholar] [CrossRef] [Green Version]
- Junold, M. Untersuchung der Eignung von Bipolartransistor und MOSFET (Metall-Oxid-Feldeffekttransistor) zur Identifikation von Kurzkettigen Carbonsäuren. Master’s Thesis, IHI Zittau/TU Dresden, Zittau, Germany, 2021. [Google Scholar]
- Giroux, S.; Rubini, P.; Aury, S. Complexes of praseodymium(III) with d-gluconic acid. Polyhedron 2000, 17, 422–428. [Google Scholar] [CrossRef]
- Gutmann, V.; Peychal-Heiling, G. Das polarographische Verhalten von Europium in Abhängigkeit vom Lösungsmittel und Leitsalz. Mon. Chem. 1969, 100, 1423–1433. [Google Scholar] [CrossRef]
- Couffin, F. Potentiels d´Oxydo-Reduction des Elements Lanthanides et Actinides dans les Solvants Organiques; CEA-BIB 233; 1980. [Google Scholar]
- Farkas, E.; Enyedy, E.A.; Zekany, L.; Deak, G. Fe hydroxamatocomplexes, natural: Interaction between iron(II) and hydroxamic acids: Oxidation of iron(II) to iron(III) by desferrioxamine B under anaerobic conditions. J. Inorg. Biochem. 2001, 83, 107–114. [Google Scholar] [CrossRef]
- Holleck, L. Zur Komplexchemie der seltenen Erden. Die polarographischen Strompotentialkurven des Europiums als Nachweismittel komplexer Bindung. Z. für Naturf. B 1947, 2, 81–89. [Google Scholar] [CrossRef]
- Blind, F. (IHI (International Institute) Zittau, TU Dresden, Zittau, Germany); Fränzle, S. (IHI (International Institute) Zittau, TU Dresden, Zittau, Germany). Influence of CH bond energies and substrate ionization potentials, orbital sequences on the chance to accomplish photooxidation by Eu(III). Unpublished results. 2019. [Google Scholar]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Markert, B.; de Li, Z. Natural background concentrations of rare-earth elements in a forest ecosystem. Sci. Total Environ. 1991, 103, 27–35. [Google Scholar] [CrossRef]
- Köllner, K.E.; Carstens, D.; Keller, E.; Vazquez, F.; Schubert, C.J.; Zeyer, J.; Bürgmann, H. Bacterial chitin hydrolysis in two lakes with contrasting trophic statuses. Appl. Environ. Microbiol. 2012, 78, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, N.R.; Mukherjee, S.K. Photosynthesis of amino acids in vitro. Nature 1934, 134, 499. [Google Scholar] [CrossRef]
- Anonymous. NIST Table of Chemicals Properties. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=116-09-6 (accessed on 15 January 2022).
- Klapiszewski, Ł.; Wysokowski, M.; Majchrzak, I.; Szatkowski, T.; Nowacka, M.; Siwińska-Stefańska, K.; Szwarc-Rzepka, K.; Bartczak, P.; Ehrlich, H.; Jesionowski, T. Preparation and Characterization of Multifunctional Chitin/Lignin Materials. J. Nanomat. 2013, 2013, 425726. [Google Scholar] [CrossRef] [Green Version]
- Hennig, H.; Rehorek, D. Photochemische und Photophysikalische Reaktionen von Koordinationsverbindungen; Deutscher Verlag der Wissenschaften: Berlin, Germany, 1987. [Google Scholar]
- Horvath, O.; Stevenson, K.L. Charge Transfer Photochemistry of Coordination Compounds; VCH: New York, NY, USA; Weinheim, Germany, 1993. [Google Scholar]
- Araki, K.; Sakuma, M.; Shiraishi, S. Photooxidation of d-fructose with iron(III) chloride under aerobic conditions. Chem. Lett. 1983, 665–666. [Google Scholar] [CrossRef] [Green Version]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton Univ. Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Wagner, G.P.; Lo, J.; Laine, R.; Almeder, M. Chitin in the epidermal cuticle of a vertebrate (Paralipophrys trigloides, Blenniidae, Teleostei). Experientia 1993, 49, 317–319. [Google Scholar] [CrossRef]
- Weiss, M.; Simon, M. Consumption of labile dissolved organic matter by limnetic bacterioplankton: The relative significance of amino acids and carbohydrates. Aquat. Microb. Ecol. 1999, 17, 1–12. [Google Scholar]
- Ruetschi, P.; Delahay, P. Hydrogen overvoltage and electrode material. A theoretical analysis. J. Chem. Phys. 1955, 23, 195–199. [Google Scholar] [CrossRef]
- Digby, P.S.B. Semi-conduction and electrode processes in biological material. Proc. Royal Soc. B 1964, 161, 504–525. [Google Scholar]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 97, 165–195. [Google Scholar] [CrossRef]
- Funahashi, R.; Ono, Y.; Qi, Z.-D.; Saito, T.; Isogai, A. Molar Masses and Molar Mass Distributions of Chitin and Acid-Hydrolyzed Chitin. Biomacromolecules 2017, 18, 4357–4363. [Google Scholar] [CrossRef]
- Ozaki, T.; Kimura, T.; Yoshida, Z.; Francis, A.J. Speciation study of actinides and lanthanides by time-resolved laser-induced fluorescence spectroscopy. Chem. Lett. 2003, 32, 560–561. [Google Scholar] [CrossRef]
- Stankiewicz, B.A.; Mastalerz, M.; Hof, C.H.J.; Bierstedt, A.; Flannery, M.B.; Briggs, D.E.G.; Evershed, R.P. Biodegradation of the chitin-protein complex in crustacean cuticle. J. Org. Geochem. 1998, 28, 67–76. [Google Scholar] [CrossRef]
- Ozaki, T.; Kimura, T.; Ohnuki, T.; Kirishima, A.; Yoshida, T.; Isobe, H.; Francis, A.J. Association of europium(III), americium(III), and curium(III) with cellulose, chitin, and chitosan. Environm. Toxicol. Chem. 2006, 25, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Pati, A.; Bhattacharyya, A.; Pujari, P.K.; Pal, S.; Kundu, T.K. Investigation of lanthanide complexation with acetohydroxamic acid in nitrate medium: Experimental and DFT studies. J. Chem. Sci. 2021, 133, 60. [Google Scholar] [CrossRef]
- Nozaki, Y. A Fresh Look at Element Distribution in the North Pacific. EOS Trans. 1997, 78, 221–226. [Google Scholar]
- Adin, A.; Sykes, A.G. The Kinetics of the oxidation of europium(II) with vanadium(III) and chromium(III) in aqueous perchloric acid solutions. J. Chem. Soc. 1966, 1230–1236. [Google Scholar] [CrossRef]
- AAdegite; Egboh, H. Kinetics and Mechanism of the Oxidation of Europium(II) by Aqueous Solution of Iodine and Triiodide. Inorg. Chim. Acta 1977, 21, 1–4. [Google Scholar] [CrossRef]
- Scheich, H.; Langner, G.; Tidemann, C.; Coles, R.B.; Guppy, A. Electroreception and electrolocation in platypus. Nature 1986, 319, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wen, Z.; Xu, Y.; Yang, H.; Lyu, L.; Zhao, Y.; Fang, C.; Shang, Y.; Du, J. Dissolved carbon in a large variety of lakes across five limnetic regions in China. J. Hydrol. 2018, 563, 143–154. [Google Scholar] [CrossRef]
- Kim, J.I.; Klenze, R.; Wimmer, H.; Runde, W.; Hauser, W. A study of the carbonate complexation of Cm(III) and Eu(III) by time-resolved laser fluorescence spectroscopy. J. Alloy. Compd. 1994, 213, 333–340. [Google Scholar] [CrossRef]
- Pol, A.; Barends, T.R.M.; Dietl, A.; Khadem, A.F.; Eygensteyn, J.; Jetten, M.S.M.; den Camp, H.J.M.O. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ. Microbiol. 2014, 16, 255–261. [Google Scholar] [CrossRef]
- Cook, E.C.; Featherston, E.R.; Showalter, S.A.; Cotruvo, J.A., Jr. Structural basis for rare earth element recognition by Methylobacterium extorquens lanmodulin. Biochemistry 2019, 58, 120–125. [Google Scholar] [CrossRef]
- Monsallier, J.-M.; Scherbaum, F.J.; Buckau, G.; Frimmel, F.H. Influence of photochemical reactions on the complexation of humic acid with europium(III). J. Photochem. Photobiol. A Chem. 2001, 138, 55–56. [Google Scholar] [CrossRef]
- Kitching, R.L. Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Annu. Rev. Entomol. 2001, 46, 729–760. [Google Scholar] [CrossRef]
- Maleke, M.; Valverde, A.; Vermeulen, J.-G.; Cason, E.; Gomez-Arias, A.; Moloantoa, K.; Coetsee-Hugo, L.; Swart, H.; van Heerden, E.; Castillo, J. Biomineralization and bioaccumulation of europium by a thermophilic metal resistant bacterium. Front. Microbiol. 2019, 10, 81–88. [Google Scholar] [CrossRef]
- Ridley, W.P.; Dizikes, L.; Cheh, A.; Wood, J.M. Recent studies on biomethylation and demethylation of toxic elements. Environ. Health Perspect. 1977, 19, 43–46. [Google Scholar] [CrossRef]
- Michalke, K.; Schmidt, A.; Huber, B.; Meyer, J.; Sulkowski, M.; Hirner, A.V.; Boertz, J.; Mosel, F.; Dammann, P.; Hilken, G. Role of intestinal microbiota in transformation of bismuth and other metals and metalloids into volatile methyl and hydride derivatives in humans and mice. Appl. Environ. Microbiol. 2008, 74, 3069–3075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddington, N.C. Nichtwässrige Lösungsmittel; utb: Heidelberg, Germany, 1972. [Google Scholar]
- Golub, A.A.; Köhler, H. Pseudohalogenide; Deutscher Verlag der Wissenschaften: Berlin, Germany, 1978. [Google Scholar]

















| Equipment Item/Substance | Precise Denomination | Supplier | Remarks |
|---|---|---|---|
| Multimeter | Peaktech 3430 | Peaktech Ahrensburg, Germany | Recording photocurrents and voltages |
| Cyclic voltammetry gear | Radiometer Solartron 402 | Tacussel Lyon, France | |
| FTIR spectrometer | Tensor 27 | Bruker Karlsruhe, Germany | |
| Electrode materials | Cu sheet | unspecified | Electrolytic Cu quality, purchased from local retailer |
| Ta wire | American Elements, Los Angeles, CA, USA | We used Ta wire or sheet electrodes to avoid corrosion observed with Cu wire grids in chitin wraps with a couple of substrates and Eu(III) | |
| Chitin | Chitin washed | Sigma-Aldrich St.Louis, MO, USA | From marine shrimp Pandalus borealis |
| Eu salts | Eu(III) trifluoromethanesulfonate | Sigma-Aldrich St.Louis, MO, USA | Nitrate or perchlorate also worked |
| Solvent | Dimethylformamide | VWR Darmstadt, Germany | |
| Microbes producing substrates | Baker´s yeast | Local retailer | Does convert mannose and others into compounds more reactive towards Eu(III)*, e.g., ethanol |
| Organic substrates of photooxidation | Glucose | ||
| Ethanolamine | Sigma-Aldrich St.Louis, MO, USA | ||
| Malonic acid | Sigma-Aldrich | Deuterated isotopomers produce far larger photocurrents | |
| Ethanediol | St.Louis, MO, USA | Deuterated isotopomers produce far larger photocurrents | |
| Glycerol | Sigma-Aldrich | Deuterated isotopomers produce far larger photocurrents | |
| Glycine | Laborchemie Apolda, Apolda, Germany Merck, Darmstadt, Germany | Partial photodecomposition (disproportionation) into ethanolamine, CO2, and CN− | |
| N-methylglycine | Sigma-Aldrich St.Louis, MO, USA | = sarcosine; does photooxidize only when kept in matrix | |
| Mannose | Sigma-Aldrich | Little phototransformation except when in matrix | |
| Mannose-1,5-diphosphate | St.Louis, MO, USA | ||
| Ribose-5-phosphate | Sigma-Aldrich | Unsubstituted ribose is photoinert with Eu(III) |
| Site, Element Ratios | Water | Antennae | Carapace | Mandi-bles | Leg Tips (Usually Immersed into Sediment) | Telson (Lower Part) | Branching Ratio on (Grafted) Chitin for Eu, M′ (Sedim.-/Water-Exposed Chitin) |
|---|---|---|---|---|---|---|---|
| Irmerteich (“Irmer´s pond”), former lignite open pit, 6.3 m deep | Eu: no data | ||||||
| Eu/La | <2.9 <2.65 | 5.19 4.74 | 0.78 0.71 | 5.51 5.04 | 4.2 3.84 | 12.7 11.6 | La 0.39 |
| Eu/Sm | <3.46 <3.42 | 16.2 16.0 | 5.8 5.74 | Sm: no data | |||
| Eu/Bi | <0.75 <1.03 | 6.3 8.66 | 11.6 15.95 | 20.3 27.9 | 12.2 16.8 | 1.03 1.41 | Bi 1.65 |
| Hartauer Lache (“shallow pond located at Zi-Hartau”); shallow pool rich in SO42−; bottom covered by clay | Eu: no data | ||||||
| Eu/La | <0.8 <0.7 | 2.56 2.34 | La 10.9 | ||||
| Eu/Sm | <1.64 <1.62 | 4.82 4.77 | Sm | ||||
| Eu/Bi | <3.4 <4.7 | 3.76 5.17 | Bi 1.9 | ||||
| Landwasser creek | Eu: data for sedim. Only | ||||||
| Eu/La | <0.94 <0.86 | 59.7 54.6 | 10.4 9.51 | 25.5 23.3 | 11.8 10.8 | La 10 | |
| Eu/Sm | <1.35 <1.34 | 39.1 38.7 | Sm: data for sedim. Only | ||||
| Eu/Bi | <90 <124 | 1.42 1.95 | 6.14 8.44 | 7.4 10.2 | 6.20 8.53 | Bi 2.2 | |
| Ostritz flooded quarry; chitin was treated with boiling water prior to analysis | Eu | ||||||
| Eu/La | <2.5 <2.3 | 14.5 13.25 | 4.61 4.21 | 14.2 13.0 | La 4.5 | ||
| Eu/Sm | <4.8 <4.75 | 80.0 79.2 | 9.6 9.5 | 36.5 36.1 | Sm 9.2 | ||
| Eu/Bi | <22.5 <30.9 | 11.7 16.1 | 7.1 9.8 | 9.6 13.2 | Bi 1.13 |
| Substance (Biopolymer) | L | σsidechain | ΔnH2O |
|---|---|---|---|
| cellulose, alkalinic conditions | O− | −0.8 | ≤2 |
| chitosan | NH2 | −0.66 | about 2 |
| cellulose | OH | −0.37 | 3–3.5 |
| chitin | NH-COCH3 | 0 | 6–6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fränzle, S.; Blind, F. Reversible Metal Ion/Complex Binding to Chitin Controlled by Ligand, Redox, and Photochemical Reactions and Active Movement of Chitin on Aquatic Arthropods. Polysaccharides 2022, 3, 515-543. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3030031
Fränzle S, Blind F. Reversible Metal Ion/Complex Binding to Chitin Controlled by Ligand, Redox, and Photochemical Reactions and Active Movement of Chitin on Aquatic Arthropods. Polysaccharides. 2022; 3(3):515-543. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3030031
Chicago/Turabian StyleFränzle, Stefan, and Felix Blind. 2022. "Reversible Metal Ion/Complex Binding to Chitin Controlled by Ligand, Redox, and Photochemical Reactions and Active Movement of Chitin on Aquatic Arthropods" Polysaccharides 3, no. 3: 515-543. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3030031