The Evaluation of the Detection of Cr(VI) in Leather
Abstract
:1. Introduction
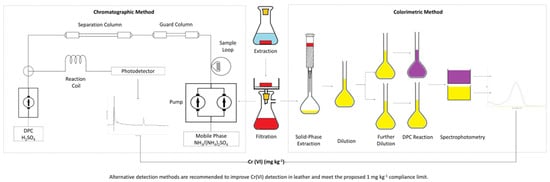
2. Materials and Methods
- Eluent system: 25 mmol (NH4)2SO4 (99%, Fisher Scientific, Cumbria, UK), 1 mmol NaOH (99%, Sigma Aldrich, Cumbria, UK);
- Column temperature: 30 °C;
- UV spectrum range: 200–500 nm;
- Extracted chromatogram wavelength: 372 nm;
- Flow rate: 1 mL min−1;
- Injection volume: 50 µL;
- Run time: 15 min.
- Eluent system: 125 mmol (NH4)2SO4, 5 mmol NaOH;
- Post-column reagent: 2 mmol 1,5-diphenylcarbazide in acidified 10% methanol solution;
- Column temperature: 30 °C;
- UV spectrum range: 200–700 nm;
- Extracted chromatogram wavelength: 540 nm;
- Flow rate: 1 mL min−1;
- Post-column reagent flow rate: 0.33 mL min−1;
- Injection volume: 50 or 100 µL;
- Run time: 7 min.
3. Results
- Oxidation of Cr(III) during extraction at pH 8.
- Reduction of Cr(VI) during extraction in the presence of a protein material.
- Dyes contributing to the absorbance at 540 nm.
- Interference from overlapping Cr(III) absorbances [17].
4. Discussion
4.1. Implications
4.2. Recommendations
- The addition of an inconclusive boundary as used in BS EN ISO 62321-7-1:2015 for mean values close to the compliance limit, where error measurements straddle the pass–fail boundary, is recommended. This could work by stipulating results are inconclusive, where errors in mean Cr(VI) values overlap with the compliance limit. Samples that fall under this category could be then tested further until a definitive pass or fail can be established.
- As Cr(III) is a significant interference for quantifying Cr(VI) and needs to be separated, it is recommended the colorimetric method is phased out as a suitable procedure for quantifying Cr(VI) in leather at 3 or 1 mg kg−1.
- To minimise the risk of false positives results due to colourants, reporting the UV-visible spectrum of the analyte and confirmation of the presence of Cr(VI) should be made mandatory.
- It is recommended that the quantity of detectable Cr(VI) in solution be maximised by using the largest injection volumes possible ideally greater than 100 µL, using larger leather sample weights, and minimising dilution factors.
- It is recommended that the absorptivity coefficient be maximised by reacting eluted Cr(VI) with DPC as a follow-up reaction during IC rather than by directly detecting the CrO42− ion. In this case, a reaction time of greater than 300 s is recommended to maximise and stabilise the colour intensity. A quoted molar absorptivity coefficient is recommended to confirm that all calibrations are correct; however, there is currently no agreed value [25]. The speciation of the of the Cr(III)-carbazone complex is currently unconfirmed; therefore, an accurate molar value cannot be derived until speciation is established [26].
- The use of a DAD and reporting the UV-visible spectrum of the analytes should be made compulsory to avoid false positives from interfering colourants.
- The LOQ for the chromatographic method should be investigated further with substantial interlaboratory trials to objectively confirm the quantification limit, as demonstrated in BS EN ISO 17075-1:2017 annex B.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Covington, A.D.; Wise, W.R. Tanning Chemistry: The Science of Leather, 2nd ed.; RSC: London, UK, 2019; Chapter 10; pp. 224–242. [Google Scholar]
- Thomson, R.S. Chrome tanning in the nineteenth century. J. Soc. Leather Technol. Chem. 1985, 69, 93–98. [Google Scholar]
- Hedberg, Y.S.; Lidén, C.; Wallinder, I.O. Correlation between bulk-and surface chemistry of Cr-tanned leather and the release of Cr(III) and Cr(VI). J. Hazard. Mater. 2014, 280, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Jing, C.; Nan, Z.; Wuyong, C.; Shiyu, S. Controlling Cr(VI) in leather: A review from passive prevention to stabilization of chromium complexes. J. Am. Leather Chem. Assoc. 2017, 112, 250–257. [Google Scholar]
- Ozkan, C.K.; Ozgunay, H.; Kalender, D. Determination of Antioxidant Properties of Commonly Used Vegetable Tannins and Their Effects on Prevention of Cr(VI) Formation. J. Soc. Leather Technol. Chem. 2015, 99, 245–249. [Google Scholar]
- Alvarez, C.C.; Bravo Gómez, M.E.; Hernández Zavala, A. Hexavalent chromium: Regulation and health effects. J. Trace Elem. Med. Biol. 2021, 65, 126729–126737. [Google Scholar] [CrossRef]
- Buters, J.; Biedermann, T. Chromium(VI) contact dermatitis: Getting closer to understanding the underlying mechanisms of toxicity and sensitization! J. Investig. Dermatol. 2017, 137, 274–277. [Google Scholar] [CrossRef] [Green Version]
- Bregnbak, D.; Johansen, J.D.; Jellesen, M.S.; Zachariae, C.; Menne, T.; Thyssen, J.P. Chromium allergy and dermatitis: Prevalence and main findings. Contact Dermat. 2015, 73, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Moretto, A. Hexavalent and trivalent chromium in leather: What should be done? Regul. Toxicol. Pharmacol. 2015, 73, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, J. Chrome Balance in Leather Processing; UNIDO: Vienna, Austria, 2000. [Google Scholar]
- BS EN ISO 17075-1:2017, British Standards Institute. Available online: https://0-shop-bsigroup-com.brum.beds.ac.uk/products/leather-chemical-determination-of-chromiumvi-content-in-leather-colorimetric-method/standard (accessed on 10 December 2021).
- BS EN ISO 17075-2:2017, British Standards Institute. Available online: https://www.mystandards.biz/standard/bseniso-17075-2-2017-28.2.2017.html (accessed on 10 December 2021).
- The European Chemicals Agency (ECHA). Available online: https://echa.europa.eu/documents/10162/1f775bd4-b1b0-4847-937f-d6a37e2c0c98 (accessed on 10 October 2021).
- The European Chemicals Agency (ECHA). Available online: https://echa.europa.eu/documents/10162/fc80f2eb-1d47-f82a-2be5-b90cdcdb5db5 (accessed on 10 October 2021).
- Ogata, K.; Kumazawa, Y.; Koyama, Y.; Yoshimura, K.; Takahashi, K. Measurement of hexavalent chromium in chrome-tanned leather: Comparative study of acidic extraction with alkaline extraction. J. Soc. Leather Technol. Chem. 2015, 99, 293–296. [Google Scholar]
- Long, A.J.; Cory, N.J.; Wood, C.B. Potential chemical mechanisms causing false positive results in hexavalent chromium determination. J. Soc. Leather Technol. Chem. 2000, 84, 74–78. [Google Scholar]
- Soares, R.; Carneiro, M.C.; Monteiro, M.I.C.; de Souza Henrique, S.; Pontes, F.V.M.; da Silva, L.I.D.; Neto, A.A.; Santelli, R.E. Simultaneous speciation of chromium by spectrophotometry and multicomponent analysis. Chem. Speciat. Bioavailab. 2009, 21, 153–160. [Google Scholar] [CrossRef]
- Rotzinger, F.P.; Stünzi, H.; Marty, W. Early stages of the hydrolysis of chromium(III) in aqueous solution. 3. Kinetics of dimerization of the deprotonated aqua ion. Inorg. Chem. 1986, 25, 489–495. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised pourbaix diagrams for chromium at 25–300 °C. Corros. Sci. 1997, 39, 43–57. [Google Scholar] [CrossRef]
- Bensalem, A.; Weckhuysen, B.M.; Schoonheydt, R.A. In Situ Diffuse Reflectance Spectroscopy of Supported Chromium Oxide Catalysts: Kinetics of the Reduction Process with Carbon Monoxide. J. Phys. Chem. B 1997, 101, 2824–2829. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Hachair, A.; Hofmann, A. Hexavalent chromium quantification in solution: Comparing direct UV–visible spectrometry with 1, 5-diphenylcarbazide colorimetry. Comptes Rendus Chim. 2018, 21, 890–896. [Google Scholar] [CrossRef]
- Fontaine, M.; Blanc, N.; Cannot, J.-C.; Demesmay, C. Ion Chromatography with Post Column Derivatization for the Determination of Hexavalent Chromium in Dyed Leather. Influence of the Preparation Method and of the Sampling Location. J. Am. Leather Chem. Assoc. 2017, 112, 319–326. [Google Scholar]
- Kral, I.; Buljan, J. The Framework for Sustainable Leather Manufacture, 2nd ed.; UNIDO: Vienna, Austria, 2019. [Google Scholar]
- Demertzis, M.A. Low detection limit spectrophotometry. Anal. Chim. Acta 2004, 505, 73–76. [Google Scholar] [CrossRef]
- Pflaum, R.T.; Howick, L.C. The Chromium-Diphenylcarbazide Reaction1. J. Am. Chem. Soc. 1956, 78, 4862–4866. [Google Scholar] [CrossRef]
- Jeffery, P.G.; Hutchison, D. Chemical Methods of Rock Analysis, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 1981; Chapter 17; pp. 159–166. [Google Scholar]
- UOP 1031-19, ASTM International. Available online: https://www.mystandards.biz/standard/uop-1031-19-1.5.2019.html (accessed on 10 December 2021).








| Detection Method | 3 mg kg−1 Cr(VI) in Leather | 1 mg kg−1 Cr(VI) in Leather | ||
|---|---|---|---|---|
| Cr(VI) in Solution (mg L−1) | Cr(VI) in Solution (µM) | Cr(VI) in Solution (mg L−1) | Cr(VI) in Solution (µM) | |
| Colorimetric Method | 0.010 | 0.18 | 0.003 | 0.06 |
| Direct Chromatographic Method | 0.061 | 1.18 | 0.021 | 0.41 |
| DPC Chromatographic Method (100 µL) | 0.059 | 1.13 | 0.019 | 0.36 |
| DPC Chromatographic Method (50 µL) | 0.062 | 1.19 | 0.022 | 0.42 |
| Analytical Method | Average LOQ (mg kg−1) | Error LOQ (mg kg−1) |
|---|---|---|
| Colorimetric | 6.50 | 0.81 |
| Chromatographic | 4.15 | 3.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, S.J.; Wise, W.R.; Recchia, S.; Spinazzè, A.; Masi, M. The Evaluation of the Detection of Cr(VI) in Leather. Analytica 2022, 3, 1-13. https://0-doi-org.brum.beds.ac.uk/10.3390/analytica3010001
Davis SJ, Wise WR, Recchia S, Spinazzè A, Masi M. The Evaluation of the Detection of Cr(VI) in Leather. Analytica. 2022; 3(1):1-13. https://0-doi-org.brum.beds.ac.uk/10.3390/analytica3010001
Chicago/Turabian StyleDavis, Stefan John, William Robert Wise, Sandro Recchia, Andrea Spinazzè, and Maurizio Masi. 2022. "The Evaluation of the Detection of Cr(VI) in Leather" Analytica 3, no. 1: 1-13. https://0-doi-org.brum.beds.ac.uk/10.3390/analytica3010001