Rare TERT Promoter Mutations Present in Benign and Malignant Cutaneous Vascular Tumors
Abstract
:1. Introduction
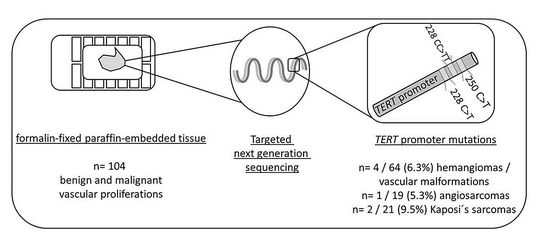
2. Materials and Methods
2.1. Sample Selection
2.2. DNA Isolation
2.3. Targeted Sequencing
3. Results
3.1. Mutation Analysis for TERT Promoter Mutations
3.2. Associations of Clinical and Pathological Parameters with TERT Promoter Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bell, R.J.; Rube, H.T.; Xavier-Magalhaes, A.; Costa, B.M.; Mancini, A.; Song, J.S.; Costello, J.F. Understanding TERT Promoter Mutations: A Common Path to Immortality. Mol. Cancer Res. 2016, 14, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent TERT promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Qu, S.; Liu, R.; Sheng, C.; Shi, X.; Zhu, G.; Murugan, A.K.; Guan, H.; Yu, H.; Wang, Y.; et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, E1130–E1136. [Google Scholar] [CrossRef] [Green Version]
- Rusinek, D.; Pfeifer, A.; Krajewska, J.; Oczko-Wojciechowska, M.; Handkiewicz-Junak, D.; Pawlaczek, A.; Zebracka-Gala, J.; Kowalska, M.; Cyplinska, R.; Zembala-Nozynska, E.; et al. Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients. Int. J. Mol. Sci. 2018, 19, 2647. [Google Scholar] [CrossRef] [Green Version]
- Hosen, I.; Rachakonda, P.S.; Heidenreich, B.; de Verdier, P.J.; Ryk, C.; Steineck, G.; Hemminki, K.; Kumar, R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer. Int. J. Cancer 2015, 137, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Spiegl-Kreinecker, S.; Lotsch, D.; Neumayer, K.; Kastler, L.; Gojo, J.; Pirker, C.; Pichler, J.; Weis, S.; Kumar, R.; Webersinke, G.; et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro. Oncol. 2018, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; Shen, Y.; Hu, D.; He, W.; Zhou, J.; Cao, Y.; Mao, Y.; Dou, Y.; Xiong, W.; Xiao, Q.; et al. Co-existence of BRAF(V600E) and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag. Res. 2018, 10, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.E.; Woo, S.R.; Nam, H.; Nam, D.H.; Lee, J.H.; Joo, K.M. Preclinical and clinical implications of TERT promoter mutation in glioblastoma multiforme. Oncol. Lett. 2017, 14, 8213–8219. [Google Scholar] [CrossRef]
- Campos, M.A.; Macedo, S.; Fernandes, M.; Pestana, A.; Pardal, J.; Batista, R.; Vinagre, J.; Sanches, A.; Baptista, A.; Lopes, J.M.; et al. TERT promoter mutations are associated with poor prognosis in cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2019, 80, 660–669 e666. [Google Scholar] [CrossRef] [PubMed]
- Malkki, H. Neuro-oncology: TERT promoter mutations could indicate poor prognosis in glioblastoma. Nat. Rev. Neurol. 2014, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Blateau, P.; Coyaud, E.; Laurent, E.; Beganton, B.; Ducros, V.; Chauchard, G.; Vendrell, J.A.; Solassol, J. TERT Promoter Mutation as an Independent Prognostic Marker for Poor Prognosis MAPK Inhibitors-Treated Melanoma. Cancers 2020, 12, 2224. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Puig-Butille, J.A.; Schilling, B.; Livingstone, E.; Potrony, M.; Carrera, C.; Schimming, T.; Moller, I.; Schwamborn, M.; et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.; Cruvinel-Carloni, A.; de Carvalho, A.C.; Sorroche, B.P.; Carvalho, A.L.; Scapulatempo-Neto, C.; Reis, R.M. TERT Promoter Mutation C228T Increases Risk for Tumor Recurrence and Death in Head and Neck Cancer Patients. Front. Oncol. 2020, 10, 1275. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 2015, 347, 1006–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, K.; Lorbeer, F.K.; Shain, A.H.; McSwiggen, D.T.; Schruf, E.; Oh, A.; Ryu, J.; Darzacq, X.; Bastian, B.C.; Hockemeyer, D. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 2017, 357, 1416–1420. [Google Scholar] [CrossRef] [Green Version]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [Green Version]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.; Pincus, L.; Ruben, B.; et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef]
- Griewank, K.G.; Murali, R.; Schilling, B.; Schimming, T.; Moller, I.; Moll, I.; Schwamborn, M.; Sucker, A.; Zimmer, L.; Schadendorf, D.; et al. TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PLoS ONE 2013, 8, e80354. [Google Scholar] [CrossRef]
- Scott, G.A.; Laughlin, T.S.; Rothberg, P.G. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014, 27, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, R.; Chandramohan, R.; Moller, I.; Scholz, S.L.; Berger, M.; Huberman, K.; Viale, A.; Pirun, M.; Socci, N.D.; Bouvier, N.; et al. Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget 2015, 6, 36041–36052. [Google Scholar] [CrossRef] [Green Version]
- Jansen, P.; Müller, H.; Lodde, G.C.; Zaremba, A.; Möller, I.; Sucker, A.; Paschen, A.; Esser, S.; Schaller, J.; Gunzer, M.; et al. GNA14, GNA11, and GNAQ Mutations Are Frequent in Benign but Not Malignant Cutaneous Vascular Tumors. Front. Genet. 2021, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- ISSVA Classification for Vascular Anomalies. 2018. Available online: https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf (accessed on 23 June 2021).
- Koopmans, A.E.; Ober, K.; Dubbink, H.J.; Paridaens, D.; Naus, N.C.; Belunek, S.; Krist, B.; Post, E.; Zwarthoff, E.C.; de Klein, A.; et al. Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions. Investig. Ophthalmol. Visual Sci. 2014, 55, 6024–6030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-Jimenez, M.T.; Blanco, L.; Ruano, Y.; Carrillo, R.; Santos-Briz, A.; Riveiro-Falkenbach, E.; Requena, L.; Kutzner, H.; Garrido, M.C.; Rodriguez-Peralto, J.L. TERT promoter mutation in sebaceous neoplasms. Virchows Arch. 2021. [Google Scholar] [CrossRef]
- Jansen, P.; Cosgarea, I.; Murali, R.; Moller, I.; Sucker, A.; Franklin, C.; Paschen, A.; Zaremba, A.; Brinker, T.J.; Stoffels, I.; et al. Frequent Occurrence of NRAS and BRAF Mutations in Human Acral Naevi. Cancers 2019, 11, 546. [Google Scholar] [CrossRef] [Green Version]
- Zaremba, A.; Murali, R.; Jansen, P.; Moller, I.; Sucker, A.; Paschen, A.; Zimmer, L.; Livingstone, E.; Brinker, T.J.; Hadaschik, E.; et al. Clinical and genetic analysis of melanomas arising in acral sites. Eur. J. Cancer 2019, 119, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Painter, C.A.; Jain, E.; Tomson, B.N.; Dunphy, M.; Stoddard, R.E.; Thomas, B.S.; Damon, A.L.; Shah, S.; Kim, D.; Gomez Tejeda Zanudo, J.; et al. The Angiosarcoma Project: Enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat. Med. 2020, 26, 181–187. [Google Scholar] [CrossRef]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.D.; Varela, I.; Lin, M.L.; Ordonez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010, 463, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.; Lim, J.Q.; Yeong, J.; Ravi, V.; Guan, P.; Boot, A.; Tay, T.K.Y.; Selvarajan, S.; Md Nasir, N.D.; Loh, J.H.; et al. Multiomic analysis and immunoprofiling reveal distinct subtypes of human angiosarcoma. J. Clin. Investig. 2020, 130, 5833–5846. [Google Scholar] [CrossRef]
- Koelsche, C.; Renner, M.; Hartmann, W.; Brandt, R.; Lehner, B.; Waldburger, N.; Alldinger, I.; Schmitt, T.; Egerer, G.; Penzel, R.; et al. TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J. Exp. Clin. Cancer Res. 2014, 33, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liau, J.Y.; Lee, J.C.; Tsai, J.H.; Chen, C.C.; Chung, Y.C.; Wang, Y.H. High frequency of GNA14, GNAQ, and GNA11 mutations in cherry hemangioma: A histopathological and molecular study of 85 cases indicating GNA14 as the most commonly mutated gene in vascular neoplasms. Mod. Pathol. 2019, 32, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Liau, J.Y.; Lee, J.C.; Tsai, J.H.; Chen, C.C.; Wang, Y.H.; Chung, Y.C. Thrombotic Hemangioma With Organizing/Anastomosing Features: Expanding the Spectrum of GNA-mutated Hemangiomas With a Predilection for the Skin of the Lower Abdominal Regions. Am. J. Surg. Pathol. 2020, 44, 255–262. [Google Scholar] [CrossRef]
- Ayturk, U.M.; Couto, J.A.; Hann, S.; Mulliken, J.B.; Williams, K.L.; Huang, A.Y.; Fishman, S.J.; Boyd, T.K.; Kozakewich, H.P.; Bischoff, J.; et al. Somatic Activating Mutations in GNAQ and GNA11 Are Associated with Congenital Hemangioma. Am. J. Hum. Genet. 2016, 98, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Macerola, E.; Loggini, B.; Giannini, R.; Garavello, G.; Giordano, M.; Proietti, A.; Niccoli, C.; Basolo, F.; Fontanini, G. Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Arch. 2015, 467, 177–184. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]


| Hemangioma/Vascular Malformation | All | WT | GNA Mutant | RAS Mutant | TERT-P-Mutant | p-Value * | |
| n = 64 | n = 43 | n = 16 $ | n = 2 | n = 4 $ | |||
| Mean age (years) | 53 | 54 | 48 | 60 | 57 | 0.4 | |
| Sex | Female | 28 | 20 | 5 $ | 0 | 3 $ | 0.36 |
| Male | 35 # | 22 | 10 # | 2 | 1 | ||
| Sites of involvement | head/neck | 21 | 16 | 3 $ | 1 | 2 $ | 0.06 |
| ventral trunk | 16 | 8 | 7 | 1 | 0 | ||
| dorsal trunk | 13 | 7 | 6 | 0 | 0 | ||
| upper extremity | 5 | 4 | 0 | 0 | 1 | ||
| lower extremity | 7 | 7 | 0 | 0 | 0 | ||
| LND | 2 | 1 | 0 | 0 | 1 | ||
| Angiosarcoma | All | WT | GNA Mutant | RAS Mutant | TERT-P-Mutant | p-Value * | |
| n = 19 | n = 15 | n = 0 | n = 3 | n = 1 | |||
| Mean age (years) | 67 | 65 | - | 75 | 74 | 0.4 | |
| Sex | Female | 15 | 11 | - | 3 | 1 | 1.0 |
| Male | 4 | 4 | 0 | 0 | |||
| Sites of involvement | head/neck | 8 | 6 | - | 2 | 0 | 1.0 |
| ventral trunk | 7 | 5 | - | 1 | 1 | ||
| dorsal trunk | 0 | 0 | - | 0 | 0 | ||
| upper extremity | 0 | 0 | - | 0 | 0 | ||
| lower extremity | 1 | 1 | - | 0 | 0 | ||
| LND | 3 | 3 | - | 0 | 0 | ||
| Kaposi‘s sarcoma | All | WT | GNA Mutant | RAS Mutant | TERT-P-Mutant | p-Value * | |
| n = 21 | n = 19 | n = 0 | n = 0 | n = 2 | |||
| Mean age (years) | 56 | 58 | - | - | 34 | 0.1 | |
| Sex | Female | 4 | 4 | - | - | 0 | 1.0 |
| Male | 16 ! | 14 ! | 2 | ||||
| Sites of involvement | head/neck | 2 | 2 | - | - | 0 | 0.13 |
| ventral trunk | 2 | 1 | - | - | 1 | ||
| dorsal trunk | 0 | 0 | - | - | 0 | ||
| upper extremity | 2 | 1 | - | - | 1 | ||
| lower extremity | 13 | 13 | - | - | 0 | ||
| LND | 2 | 2 | - | - | 0 | ||
| Hemangioma/Vascular Malformation | All | WT | GNA Mutant | RAS Mutant | TERT-P-Mutant |
|---|---|---|---|---|---|
| n = 64 | n = 43 | n = 16 $ | n = 2 | n = 4 $ | |
| lobular capillary/pyogenic granuloma | 7 | 3 | 3 | 1 | 0 |
| microvenular | 6 | 6 | 0 | 0 | 0 |
| cherry/senile # | 25 | 10 | 13 $ | 1 | 2 $ |
| tufted | 1 | 1 | 0 | 0 | 0 |
| Angiokeratoma + | 3 | 3 | 0 | 0 | 0 |
| Arteriovenous + | 4 | 4 | 0 | 0 | 0 |
| superficial hemosiderotic lymphovascular +x | 2 | 2 | 0 | 0 | 0 |
| venous/cavernous + | 16 | 14 | 0 | 0 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, P.; Lodde, G.C.; Zaremba, A.; Thielmann, C.M.; Matull, J.; Müller, H.; Möller, I.; Sucker, A.; Esser, S.; Schaller, J.; et al. Rare TERT Promoter Mutations Present in Benign and Malignant Cutaneous Vascular Tumors. Dermato 2021, 1, 18-25. https://0-doi-org.brum.beds.ac.uk/10.3390/dermato1010003
Jansen P, Lodde GC, Zaremba A, Thielmann CM, Matull J, Müller H, Möller I, Sucker A, Esser S, Schaller J, et al. Rare TERT Promoter Mutations Present in Benign and Malignant Cutaneous Vascular Tumors. Dermato. 2021; 1(1):18-25. https://0-doi-org.brum.beds.ac.uk/10.3390/dermato1010003
Chicago/Turabian StyleJansen, Philipp, Georg Christian Lodde, Anne Zaremba, Carl Maximilian Thielmann, Johanna Matull, Hansgeorg Müller, Inga Möller, Antje Sucker, Stefan Esser, Jörg Schaller, and et al. 2021. "Rare TERT Promoter Mutations Present in Benign and Malignant Cutaneous Vascular Tumors" Dermato 1, no. 1: 18-25. https://0-doi-org.brum.beds.ac.uk/10.3390/dermato1010003