Estimation of Avocado Oil (Persea americana Mill., Greek “Zutano” Variety) Volatile Fraction over Ripening by Classical and Ultrasound Extraction Using HS-SPME–GC–MS
Abstract
:1. Introduction
2. Materials and Methods
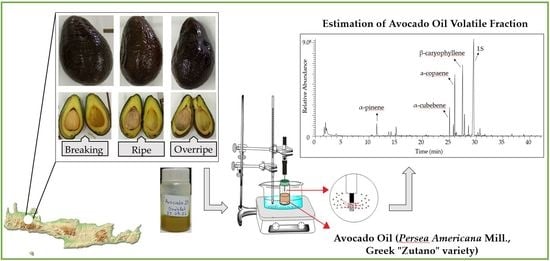
2.1. Avocado Fruit Samples
2.2. Avocado Oil Extraction
2.3. Isolation and Analysis of Avocado Oil Volatile Fraction
2.4. Statistical Analysis
3. Results and Discussion
3.1. Estimation of Avocado Oil Yield
3.2. Volatile Compounds Analysis
3.3. Estimation of Volatiles over Extraction Method
3.4. Estimation of Volatiles over Ripening
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caballero, B.; Finglas, P.M.; Toldrá, F. (Eds.) Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; ISBN 978-0-12-384947-2. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 24 November 2021).
- Kourgialas, N.N.; Dokou, Z. Water management and salinity adaptation approaches of Avocado trees: A review for hot-summer Mediterranean climate. Agric. Water Manag. 2021, 252, 106923. [Google Scholar] [CrossRef]
- Flores, M.; Saravia, C.; Vergara, C.; Avila, F.; Valdés, H.; Ortiz-Viedma, J. Avocado Oil: Characteristics, Properties, and Applications. Molecules 2019, 24, 2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.X. Virgin avocado oil: An emerging source of functional fruit oil. J. Funct. Foods 2019, 54, 381–392. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Paz, B.; Yahia, E.M. Avocado oil: Production and market demand, bioactive components, implications in health, and tendencies and potential uses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4120–4158. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.; Wong, M.; Eyres, L.; McGhie, T.; Lund, C.; Olsson, S.; Wang, Y.; Bulley, C.; Wang, M.; Friel, E.; et al. Avocado oil. In Gourmet and Health-Promoting Specialty Oils; Elsevier: Amsterdam, The Netherlands, 2009; pp. 73–125. ISBN 978-1-893997-97-4. [Google Scholar]
- El-Zeftawi, B. Physical and chemical changes in fruit of seven avocado cultivars at Mildura. Aust. J. Agric. Res. 1978, 29, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Vekiari, S.A.; Papadopoulou, P.P.; Lionakis, S.; Krystallis, A. Variation in the Composition of Cretan Avocado Cultivars during Ripening: Seasonal Variation in the Composition of Cretan Avocados. J. Sci. Food Agric. 2004, 84, 485–492. [Google Scholar] [CrossRef]
- Takenaga, F.; Matsuyama, K.; Abe, S.; Torii, Y.; Itoh, S. Lipid and fatty acid composition of mesocarp and seed of avocado fruits harvested at northern range in Japan. J. Oleo Sci. 2008, 57, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, F.; Topuz, A. Changes in dry matter, oil content and fatty acids composition of avocado during harvesting time and post-harvesting ripening period. Food Chem. 2004, 86, 79–83. [Google Scholar] [CrossRef]
- Mahendran, T.; Brennan, J.G.; Hariharan, G. Aroma volatiles components of ‘Fuerte’ Avocado (Persea americana Mill.) stored under different modified atmospheric conditions. J. Essent. Oil Res. 2019, 31, 34–42. [Google Scholar] [CrossRef]
- Ali, S.; Plotto, A.; Scully, B.T.; Wood, D.; Stover, E.; Owens, N.; Pisani, C.; Ritenour, M.; Anjum, M.A.; Nawaz, A.; et al. Fatty acid and volatile organic compound profiling of avocado germplasm grown under East-Central Florida conditions. Sci. Hortic. 2020, 261, 109008. [Google Scholar] [CrossRef]
- Tan, C.X.; Hean, C.G.; Hamzah, H.; Ghazali, H.M. Optimization of ultrasound-assisted aqueous extraction to produce virgin avocado oil with low free fatty acids. J. Food Process. Eng. 2018, 41, e12656. [Google Scholar] [CrossRef]
- Tan, C.X.; Chong, G.H.; Hamzah, H.; Ghazali, H.M. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. J. Supercrit. Fluids 2018, 135, 45–51. [Google Scholar] [CrossRef]
- Dos Santos, M.A.Z.; Alicieo, T.V.R.; Pereira, C.M.P.; Ramis-Ramos, G.; Mendonça, C.R.B.; Dos Santos, M.A.Z. Profile of Bioactive Compounds in Avocado Pulp Oil: Influence of the Drying Processes and Extraction Methods. J. Am. Oil Chem. Soc. 2013, 91, 19–27. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Borges, C.D.; Mendonça, C.R.B.; Jansen-Alves, C.; Zambiazi, R.C. Bioactive compounds and quality parameters of avocado oil obtained by different processes. Food Chem. 2018, 257, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Moodley, R.; Jonnalagadda, S.B.; Jonnalagadda, S.B. Fatty acid profile and elemental content of avocado (Persea americana Mill. ) oil –effect of extraction methods. J. Environ. Sci. Health Part B 2012, 47, 529–537. [Google Scholar] [CrossRef]
- Abaide, E.; Zabot, G.L.; Tres, M.V.; Martins, R.F.; Fagundez, J.L.; Nunes, L.F.; Druzian, S.; Soares, J.F.; Prá, V.D.; Silva, J.R.; et al. Yield, composition, and antioxidant activity of avocado pulp oil extracted by pressurized fluids. Food Bioprod. Process. 2017, 102, 289–298. [Google Scholar] [CrossRef]
- Mostert, M.E.; Botha, B.M.; Du Plessis, L.M.; Duodu, K.G. Effect of fruit ripeness and method of fruit drying on the extractability of avocado oil with hexane and supercritical carbon dioxide. J. Sci. Food Agric. 2007, 87, 2880–2885. [Google Scholar] [CrossRef]
- Corzzini, S.C.; Barros, H.D.; Grimaldi, R.; Cabral, F. Extraction of edible avocado oil using supercritical CO2 and a CO2/ethanol mixture as solvents. J. Food Eng. 2017, 194, 40–45. [Google Scholar] [CrossRef]
- Espinosa-Alonso, L.G.; Paredes-López, O.; Valdez-Morales, M.; Oomah, B.D. Avocado Oil Characteristics of Mexican Creole Genotypes: Mexican Creole Avocado Oil Properties. Eur. J. Lipid Sci. Technol. 2017, 119, 1600406. [Google Scholar] [CrossRef]
- Yanty, N.A.M.; Marikkar, J.M.N.; Long, K. Effect of Varietal Differences on Composition and Thermal Characteristics of Avocado Oil. J. Am. Oil Chem. Soc. 2011, 88, 1997–2003. [Google Scholar] [CrossRef]
- Moreno, A.O.; Dorantes, L.; Galíndez, J.; Guzmán, R.I. Effect of Different Extraction Methods on Fatty Acids, Volatile Compounds, and Physical and Chemical Properties of Avocado (Persea americana Mill.) Oil. J. Agric. Food Chem. 2003, 51, 2216–2221. [Google Scholar] [CrossRef]
- Meyer, M.D.; Terry, L.A. Development of a Rapid Method for the Sequential Extraction and Subsequent Quantification of Fatty Acids and Sugars from Avocado Mesocarp Tissue. J. Agric. Food Chem. 2008, 56, 7439–7445. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Dorantes, A.L.; Gallndez, M.J.; CRdenas, S.E. Effect of a Novel Oil Extraction Method on Avocado (Persea americana Mill.) Pulp Microstructure. Plant Foods Hum. Nutr. 2004, 59, 11–14. [Google Scholar] [CrossRef]
- Meyer, M.D.; Terry, L.A. Fatty Acid and Sugar Composition of Avocado, Cv. Hass, in Response to Treatment with an Ethylene Scavenger or 1-Methylcyclopropene to Extend Storage Life. Food Chem. 2010, 121, 1203–1210. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Coutinho, J.P.; Grimaldi, R.; Godoy, H.T.; Cabral, F.A. Simultaneous Extraction of Edible Oil from Avocado and Capsanthin from Red Bell Pepper Using Supercritical Carbon Dioxide as Solvent. J. Supercrit. Fluids 2016, 107, 315–320. [Google Scholar] [CrossRef]
- Martínez-Padilla, L.P.; Franke, L.; Xu, X.-Q.; Juliano, P. Improved Extraction of Avocado Oil by Application of Sono-Physical Processes. Ultrason. Sonochem. 2018, 40, 720–726. [Google Scholar] [CrossRef] [PubMed]
- del Pilar Ramírez-Anaya, J.; Manzano-Hernández, A.J.; Tapia-Campos, E.; Alarcón-Domínguez, K.; Castañeda-Saucedo, M.C. Influence of Temperature and Time during Malaxation on Fatty Acid Profile and Oxidation of Centrifuged Avocado Oil. Food Sci. Technol. 2018, 38, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.; Olaeta, J.A.; Undurraga, P. Mejoramiento del rendimiento de extracción del aceite de palta (aguacate). In Proceedings of the VI World Avocado Congress (Actas VI Congreso Mundial del Aguacate) 2007, Viña Del Mar, Chile, 12–16 November 2007. [Google Scholar]
- Kilic-Buyukkurt, O. Characterization of Aroma Compounds of Cold-Pressed Avocado Oil Using Solid-Phase Microextraction Techniques with Gas Chromatography–Mass Spectrometry. J. Raw Mater. Process. Foods 2021, 2, 1–7. [Google Scholar]
- Haiyan, Z.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous Biophenol, Fatty Acid and Volatile Profiles of Selected Oils. Food Chem. 2007, 100, 1544–1551. [Google Scholar] [CrossRef]
- Pino, J.A.; Rosado, A.; Aguero, J. Volatile Components of Avocado (Persea americana Mill.) Fruits. J. Essent. Oil Res. 2000, 12, 377–378. [Google Scholar] [CrossRef]
- de Sousa Galvao, M.; Nunes, M.L.; Constant, P.B.L.; Narain, N. Identification of Volatile Compounds in Cultivars Barker, Collinson, Fortuna and Geada of Avocado (Persea americana Mill.) Fruit. Food Sci. Technol. 2016, 36, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-J.; Gong, X.; Jing, W.; Lin, L.-J.; Zhou, W.; He, J.-N.; Li, J.-H. Fast Discrimination of Avocado Oil for Different Extracted Methods Using Headspace-Gas Chromatography-Ion Mobility Spectroscopy with PCA Based on Volatile Organic Compounds. Open Chem. 2021, 19, 367–376. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 387/2005 of 8 March 2005 Amending (EC) Regulation No 831/97 Laying down Marketing Standards Applicable to Avocados—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/dd5847ac-2cc1-431f-b945-8c2ecb500f40 (accessed on 24 November 2021).
- Xagoraris, M.; Revelou, P.-K.; Dedegkika, S.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. SPME-GC-MS and FTIR-ATR Spectroscopic Study as a Tool for Unifloral Common Greek Honeys’ Botanical Origin Identification. Appl. Sci. 2021, 11, 3159. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Satriana, S.; Supardan, M.D.; Arpi, N.; Wan Mustapha, W.A. Development of Methods Used in the Extraction of Avocado Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800210. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.E.C.; Tieman, D.M.; Sargent, S.A.; Klee, H.J.; Huber, D.J. Volatile Profiles of Ripening West Indian and Guatemalan-West Indian Avocado Cultivars as Affected by Aqueous 1-Methylcyclopropene. Postharvest Biol. Technol. 2013, 80, 37–46. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C.; Agustí, M. Maturity Stage at Harvest Affects Fruit Ripening, Quality and Biosynthesis of Aroma Volatile Compounds in ‘Kensington Pride’ Mango. J. Hortic. Sci. Biotechnol. 2003, 78, 225–233. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. Aroma Volatiles Production during Fruit Ripening of ‘Kensington Pride’ Mango. Postharvest Biol. Technol. 2003, 27, 323–336. [Google Scholar] [CrossRef]
- Zidi, K.; Kati, D.E.; Bachir-bey, M.; Genva, M.; Fauconnier, M.-L. Comparative Study of Fig Volatile Compounds Using Headspace Solid-Phase Microextraction-Gas Chromatography/Mass Spectrometry: Effects of Cultivars and Ripening Stages. Front. Plant Sci. 2021, 12, 667809. [Google Scholar] [CrossRef]
- Schaffer, R.J.; Friel, E.N.; Souleyre, E.J.F.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J.H.; Ma, J.-H.; Nain, B.; Cohen, D.; et al. A Genomics Approach Reveals That Aroma Production in Apple Is Controlled by Ethylene Predominantly at the Final Step in Each Biosynthetic Pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Kovács, K.; Fray, R.G.; Tikunov, Y.; Graham, N.; Bradley, G.; Seymour, G.B.; Bovy, A.G.; Grierson, D. Effect of Tomato Pleiotropic Ripening Mutations on Flavour Volatile Biosynthesis. Phytochemistry 2009, 70, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.S.; Kulkarni, R.S.; Chidley, H.G.; Giri, A.P.; Pujari, K.H.; Köllner, T.G.; Degenhardt, J.; Gershenzon, J.; Gupta, V.S. Changes in Volatile Composition during Fruit Development and Ripening of ‘Alphonso’ Mango. J. Sci. Food Agric. 2009, 89, 2071–2081. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manríquez, D.; Luengwilai, K.; González-Agüero, M. Chapter 1 Aroma Volatiles. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2009; Volume 50, pp. 1–37. ISBN 978-0-12-374835-5. [Google Scholar]
- Gapper, N.E.; McQuinn, R.P.; Giovannoni, J.J. Molecular and Genetic Regulation of Fruit Ripening. Plant Mol. Biol. 2013, 82, 575–591. [Google Scholar] [CrossRef]
- Platt, K.A.; Thomson, W.W. Idioblast Oil Cells of Avocado: Distribution, Isolation, Ultrastructure, Histochemistry, and Biochemistry. Int. J. Plant Sci. 1992, 153, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Platt-Aloia, K.A.; Oross, J.W.; Thomson, W.W. Ultrastructural Study of the Development of Oil Cells in the Mesocarp of Avocado Fruit. Bot. Gaz. 1983, 144, 49–55. [Google Scholar] [CrossRef]



| Volatile Compounds | CAS Number | RT a | RI b | Soxhlet | UAE | ||||
|---|---|---|---|---|---|---|---|---|---|
| Breaking | Ripe | Overripe | Breaking | Ripe | Overripe | ||||
| Hydrocarbons (Non Terpenoids) | |||||||||
| 1-ethyl-2-methylcyclohexane | 3728-54-9 | 9.7 | 883 | 0.20 | 0.15 | 0.21 | 0.20 | 0.00 | 0.20 |
| (1R,3S)-1-ethyl-3-methylcyclohexane | 3728-55-0 | 9.8 | 887 | 0.33 | 0.15 | 0.19 | 0.33 | 0.01 | 0.22 |
| nonane | 111-84-2 | 10.2 | 897 | 1.34 | 0.74 | 1.62 | 1.34 | 0.04 | 1.02 |
| propylcyclohexane | 1678-92-8 | 11.2 | 922 | 0.20 | 0.06 | 0.10 | 0.20 | 0.02 | 0.30 |
| 2,6-dimethyloctane | 2051-30-1 | 11.6 | 932 | 0.93 | 0.15 | 0.85 | 0.93 | 0.00 | 0.54 |
| 3-ethyl-2-methylheptane | 14676-29-0 | 11.8 | 937 | 0.78 | 0.43 | 0.82 | 0.78 | 0.00 | 0.70 |
| 1,1,2,3-tetramethylcyclohexane | 6783-92-2 | 12.3 | 951 | 0.19 | 0.04 | 0.24 | 0.46 | 0.00 | 0.25 |
| 4-ethyloctane | 15869-86-0 | 12.4 | 953 | 0.40 | 0.16 | 0.48 | 0.40 | 0.01 | 0.36 |
| 4-methylnonane | 17301-94-9 | 12.7 | 961 | 0.74 | 0.30 | 0.95 | 0.74 | 0.07 | 0.69 |
| 2-methylnonane | 871-83-0 | 12.8 | 964 | 0.85 | 0.28 | 1.00 | 0.85 | 0.06 | 0.70 |
| 3-methylnonane | 5911-04-6 | 13.0 | 970 | 1.19 | 0.47 | 1.62 | 1.19 | 0.04 | 1.07 |
| 1-methyl-2-propylcyclohexane | 4291-79-6 | 13.6 | 986 | 0.86 | 0.32 | 1.64 | 0.86 | 0.06 | 1.32 |
| decane | 124-18-5 | 14.2 | 1001 | 4.45 | 1.80 | 6.32 | 4.47 | 0.51 | 5.08 |
| butylcyclohexane | 1678-93-9 | 15.2 | 1032 | 0.57 | 0.00 | 0.50 | 0.57 | 0.00 | 0.40 |
| dodecane | 112-40-3 | 20.6 | 1200 | 0.19 | 0.06 | 0.07 | 0.19 | 0.15 | 0.10 |
| (1S,2S,3R,4S,6R,7R,8S)-1,2-dimethyl-8-propan-2-yltetracyclo[4.4.0.02,4.03,7]decane (cyclosativene) | 22469-52-9 | 25.8 | 1369 | 0.64 | 0.81 | 0.54 | 0.64 | 1.01 | 0.62 |
| (1S,2S,4R)-1-ethenyl-1-methyl-2,4-bis(prop-1-en-2-yl)cyclohexane (β-elemene) | 515-13-9 | 26.3 | 1387 | 0.36 | 0.44 | 0.25 | 0.44 | 0.67 | 0.35 |
| tetradecane | 629-59-4 | 26.8 | 1401 | 0.10 | 0.01 | 0.10 | 0.10 | 0.08 | 0.10 |
| 10,10-dimethyl-2,6-dimethylenebicyclo[7.2.0]undecane | 136296-38-3 | 27.7 | 1427 | 0.10 | 0.20 | 0.10 | 0.10 | 0.17 | 0.13 |
| Terpenoids | |||||||||
| 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (α-pinene) | 7785-70-8 | 11.5 | 929 | 1.79 | 2.57 | 0.74 | 1.80 | 1.95 | 0.36 |
| 7-methyl-3-methyleneocta-1,6-diene (β-myrcene) | 123-35-3 | 13.7 | 988 | 0.96 | 0.94 | 0.33 | 0.96 | 0.64 | 0.39 |
| 1-methyl-4-propan-2-ylbenzene (p-cymene) | 99-87-6 | 14.9 | 1022 | 0.82 | 0.44 | 0.96 | 0.82 | 0.23 | 0.75 |
| 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene (D-limonene) | 138-86-3 | 15.1 | 1028 | 0.99 | 1.50 | 0.27 | 0.99 | 1.20 | 0.31 |
| 1-isopropyl-4-methylcyclohexa-1,4-diene (γ-terpinene) | 99-85-4 | 16.1 | 1057 | 0.00 | 0.10 | 0.00 | 0.00 | 0.11 | 0.00 |
| 1-methyl-4-(propan-2-ylidene)cyclohex-1-ene | 586-62-9 | 17.0 | 1085 | 0.15 | 0.07 | 0.04 | 0.15 | 0.07 | 0.03 |
| 1-methyl-4-(prop-1-en-2-yl)benzene (p-cymenene) | 1195-32-0 | 17.2 | 1090 | 0.09 | 0.14 | 0.01 | 0.09 | 0.14 | 0.02 |
| 4,10-dimethyl-7-propan-2-yltricyclo[4.4.0.01,5]dec-3-ene (α-cubebene) | 17699-14-8 | 25.0 | 1346 | 1.48 | 1.70 | 1.19 | 1.70 | 2.28 | 1.42 |
| (1S,6S,7S,8S)-1,3-dimethyl-8-propan-2-yltricyclo[4.4.0.02,7]dec-3-ene (a-copaene) | 3856-25-5 | 26.0 | 1377 | 3.80 | 5.27 | 3.66 | 3.81 | 7.15 | 4.24 |
| (1R,9S,Z)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene (β-caryophyllene) | 87-44-5 | 27.5 | 1422 | 5.78 | 8.07 | 4.32 | 5.78 | 9.84 | 4.73 |
| 2,6-dimethyl-6-(4-methylpent-3-enyl)bicyclo[3.1.1]hept-2-ene (α-bergamotene) | 13474-59-4 | 27.8 | 1432 | 1.05 | 1.55 | 1.09 | 1.05 | 2.24 | 1.21 |
| (6Z)-7,11-dimethyl-3-methylidenedodeca-1,6,10-triene (β-farnesene) | 28973-97-9 | 28.4 | 1450 | 0.05 | 0.07 | 0.03 | 0.05 | 0.13 | 0.08 |
| (1E,4E,8E)-2,6,6,9-tetramethylcycloundeca-1,4,8-triene (humulene) | 6753-98-6 | 28.6 | 1454 | 0.54 | 0.76 | 0.45 | 0.54 | 0.93 | 0.52 |
| (1aR,4aS,7R,7aS,7bS)-1,1,7-trimethyl-4-methylidene-2,3,4a,5,6,7,7a,7b-octahydro-1aH-cyclopropa[e]azulene (alloaromadendrene) | 25246-27-9 | 28.7 | 1458 | 0.13 | 0.16 | 0.11 | 0.13 | 0.20 | 0.14 |
| (1S,4aS,8aR)-7-methyl-4-methylidene-1-propan-2-yl-2,3,4a,5,6,8a-hexahydro-1H-naphthalene (γ-muurolene) | 30021-74-0 | 29.2 | 1473 | 0.08 | 0.15 | 0.08 | 0.08 | 0.21 | 0.12 |
| (1S,4aS,8aR)-4,7-dimethyl-1-propan-2-yl-1,2,4a,5,6,8a-hexahydronaphthalene (α-muurolene) | 31983-22-9 | 30.0 | 1497 | 0.08 | 0.10 | 0.05 | 0.05 | 0.14 | 0.09 |
| (4S)-1-methyl-4-(6-methylhepta-1,5-dien-2-yl)cyclohexene (β-bisabolene) | 495-61-4 | 30.3 | 1506 | 0.14 | 0.23 | 0.24 | 0.14 | 0.36 | 0.30 |
| (1R,4aS,8aS)-7-methyl-4-methylidene-1-propan-2-yl-2,3,4a,5,6,8a-hexahydro-1H-naphthalene (γ-cadinene) | 39029-41-9 | 30.5 | 1511 | 0.04 | 0.08 | 0.04 | 0.04 | 0.09 | 0.06 |
| (1S,8aR)-4,7-dimethyl-1-propan-2-yl-1,2,3,5,6,8a-hexahydronaphthalene (δ-cadinene) | 483-76-1 | 30.7 | 1517 | 0.40 | 0.65 | 0.51 | 0.40 | 0.66 | 0.57 |
| 4-isopropyl-1,6-dimethyl-1,2,3,4,4a,7-hexahydronaphthalene | 16728-99-7 | 31.1 | 1530 | 0.04 | 0.07 | 0.05 | 0.04 | 0.08 | 0.06 |
| (1S)-4,7-dimethyl-1-propan-2-yl-1,2-dihydronaphthalene (α-calacorene) | 21391-99-1 | 31.4 | 1538 | 0.04 | 0.08 | 0.09 | 0.05 | 0.08 | 0.09 |
| Aldehydes | |||||||||
| nonanal | 124-19-6 | 17.7 | 1104 | 0.08 | 0.11 | 0.07 | 0.08 | 0.16 | 0.02 |
| Ketones | |||||||||
| octan-2-one | 111-13-7 | 10.6 | 907 | 0.56 | 0.28 | 0.48 | 0.27 | 0.12 | 0.19 |
| Others | |||||||||
| trans-decahydronaphthalene | 493-02-7 | 16.1 | 1057 | 0.34 | 0.10 | 0.25 | 0.27 | 0.02 | 0.27 |
| (1S,4S)-1,6-dimethyl-4-propan-2-yl-1,2,3,4-tetrahydronaphthalene (calamenene) | 72937-55-4 | 30.8 | 1519 | 0.13 | 0.24 | 0.19 | 0.13 | 0.23 | 0.22 |
| Multiple Comparisons a | ||||
|---|---|---|---|---|
| No. | Volatile Compounds | Ripening Stages in Pairs | p-Value b | |
| 1 | 2,6-dimethyloctane | Breaking | Ripe | 0.021 |
| Ripe | Overripe | 0.049 | ||
| Overripe | Breaking | 0.373 | ||
| 2 | 4-methylnonane | Breaking | Ripe | 0.066 |
| Ripe | Overripe | 0.047 | ||
| Overripe | Breaking | 0.859 | ||
| 3 | 2-methylnonane | Breaking | Ripe | 0.047 |
| Ripe | Overripe | 0.047 | ||
| Overripe | Breaking | 1.000 | ||
| 4 | 1-methyl-2-propylcyclohexane | Breaking | Ripe | 0.064 |
| Ripe | Overripe | 0.011 | ||
| Overripe | Breaking | 0.077 | ||
| 5 | decane | Breaking | Ripe | 0.046 |
| Ripe | Overripe | 0.019 | ||
| Overripe | Breaking | 0.364 | ||
| 6 | butylcyclohexane | Breaking | Ripe | 0.002 |
| Ripe | Overripe | 0.004 | ||
| Overripe | Breaking | 0.131 | ||
| 7 | 10,10-dimethyl-2,6-dimethylenebicyclo[7.2.0]undecane | Breaking | Ripe | 0.037 |
| Ripe | Overripe | 0.061 | ||
| Overripe | Breaking | 0.716 | ||
| 8 | 2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (α-pinene) | Breaking | Ripe | 0.408 |
| Ripe | Overripe | 0.024 | ||
| Overripe | Breaking | 0.056 | ||
| 9 | 7-methyl-3-methyleneocta-1,6-diene (β-myrcene) | Breaking | Ripe | 0.486 |
| Ripe | Overripe | 0.091 | ||
| Overripe | Breaking | 0.039 | ||
| 10 | 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene (D-limonene) | Breaking | Ripe | 0.133 |
| Ripe | Overripe | 0.008 | ||
| Overripe | Breaking | 0.025 | ||
| 11 | 1-isopropyl-4-methylcyclohexa-1,4-diene (γ-terpinene) | Breaking | Ripe | 0.000 |
| Ripe | Overripe | 0.000 | ||
| Overripe | Breaking | 1.000 | ||
| 12 | 1-methyl-4-(propan-2-ylidene)cyclohex-1-ene | Breaking | Ripe | 0.001 |
| Ripe | Overripe | 0.008 | ||
| Overripe | Breaking | 0.000 | ||
| 13 | 1-methyl-4-(prop-1-en-2-yl)benzene (p-cymenene) | Breaking | Ripe | 0.003 |
| Ripe | Overripe | 0.000 | ||
| Overripe | Breaking | 0.001 | ||
| 14 | (1R,9S,Z)-4,11,11-trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene (β-caryophyllene) | Breaking | Ripe | 0.053 |
| Ripe | Overripe | 0.022 | ||
| Overripe | Breaking | 0.366 | ||
| 15 | (1E,4E,8E)-2,6,6,9-tetramethylcycloundeca-1,4,8-triene (humulene) | Breaking | Ripe | 0.060 |
| Ripe | Overripe | 0.039 | ||
| Overripe | Breaking | 0.781 | ||
| 16 | (1R,4aS,8aS)-7-methyl-4-methylidene-1-propan-2-yl-2,3,4a,5,6,8a-hexahydro-1H-naphthalene (γ-cadinene) | Breaking | Ripe | 0.036 |
| Ripe | Overripe | 0.070 | ||
| Overripe | Breaking | 0.604 | ||
| 17 | (1S,8aR)-4,7-dimethyl-1-propan-2-yl-1,2,3,5,6,8a-hexahydronaphthalene (δ-cadinene) | Breaking | Ripe | 0.005 |
| Ripe | Overripe | 0.043 | ||
| Overripe | Breaking | 0.025 | ||
| 18 | 4-isopropyl-1,6-dimethyl-1,2,3,4,4a,7-hexahydronaphthalene | Breaking | Ripe | 0.021 |
| Ripe | Overripe | 0.089 | ||
| Overripe | Breaking | 0.171 | ||
| 19 | (1S)-4,7-dimethyl-1-propan-2-yl-1,2-dihydronaphthalene (α-calacorene) | Breaking | Ripe | 0.008 |
| Ripe | Overripe | 0.192 | ||
| Overripe | Breaking | 0.004 | ||
| 20 | trans-decahydronaphthalene | Breaking | Ripe | 0.026 |
| Ripe | Overripe | 0.046 | ||
| Overripe | Breaking | 0.640 | ||
| 21 | (1S,4S)-1,6-dimethyl-4-propan-2-yl-1,2,3,4-tetrahydronaphthalene (calamenene) | Breaking | Ripe | 0.009 |
| Ripe | Overripe | 0.213 | ||
| Overripe | Breaking | 0.023 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xagoraris, M.; Galani, E.; Valasi, L.; Kaparakou, E.H.; Revelou, P.-K.; Tarantilis, P.A.; Pappas, C.S. Estimation of Avocado Oil (Persea americana Mill., Greek “Zutano” Variety) Volatile Fraction over Ripening by Classical and Ultrasound Extraction Using HS-SPME–GC–MS. Compounds 2022, 2, 25-36. https://0-doi-org.brum.beds.ac.uk/10.3390/compounds2010003
Xagoraris M, Galani E, Valasi L, Kaparakou EH, Revelou P-K, Tarantilis PA, Pappas CS. Estimation of Avocado Oil (Persea americana Mill., Greek “Zutano” Variety) Volatile Fraction over Ripening by Classical and Ultrasound Extraction Using HS-SPME–GC–MS. Compounds. 2022; 2(1):25-36. https://0-doi-org.brum.beds.ac.uk/10.3390/compounds2010003
Chicago/Turabian StyleXagoraris, Marinos, Eleni Galani, Lydia Valasi, Eleftheria H. Kaparakou, Panagiota-Kyriaki Revelou, Petros A. Tarantilis, and Christos S. Pappas. 2022. "Estimation of Avocado Oil (Persea americana Mill., Greek “Zutano” Variety) Volatile Fraction over Ripening by Classical and Ultrasound Extraction Using HS-SPME–GC–MS" Compounds 2, no. 1: 25-36. https://0-doi-org.brum.beds.ac.uk/10.3390/compounds2010003