Net Zero and Catalysis: How Neutrons Can Help
Abstract
:1. Introduction
2. Neutron Scattering
3. The Opportunities
3.1. Hydrogen
3.2. Methane Reforming
3.3. Ammonia
3.4. Fischer–Tropsch Synthesis
3.5. Methanol
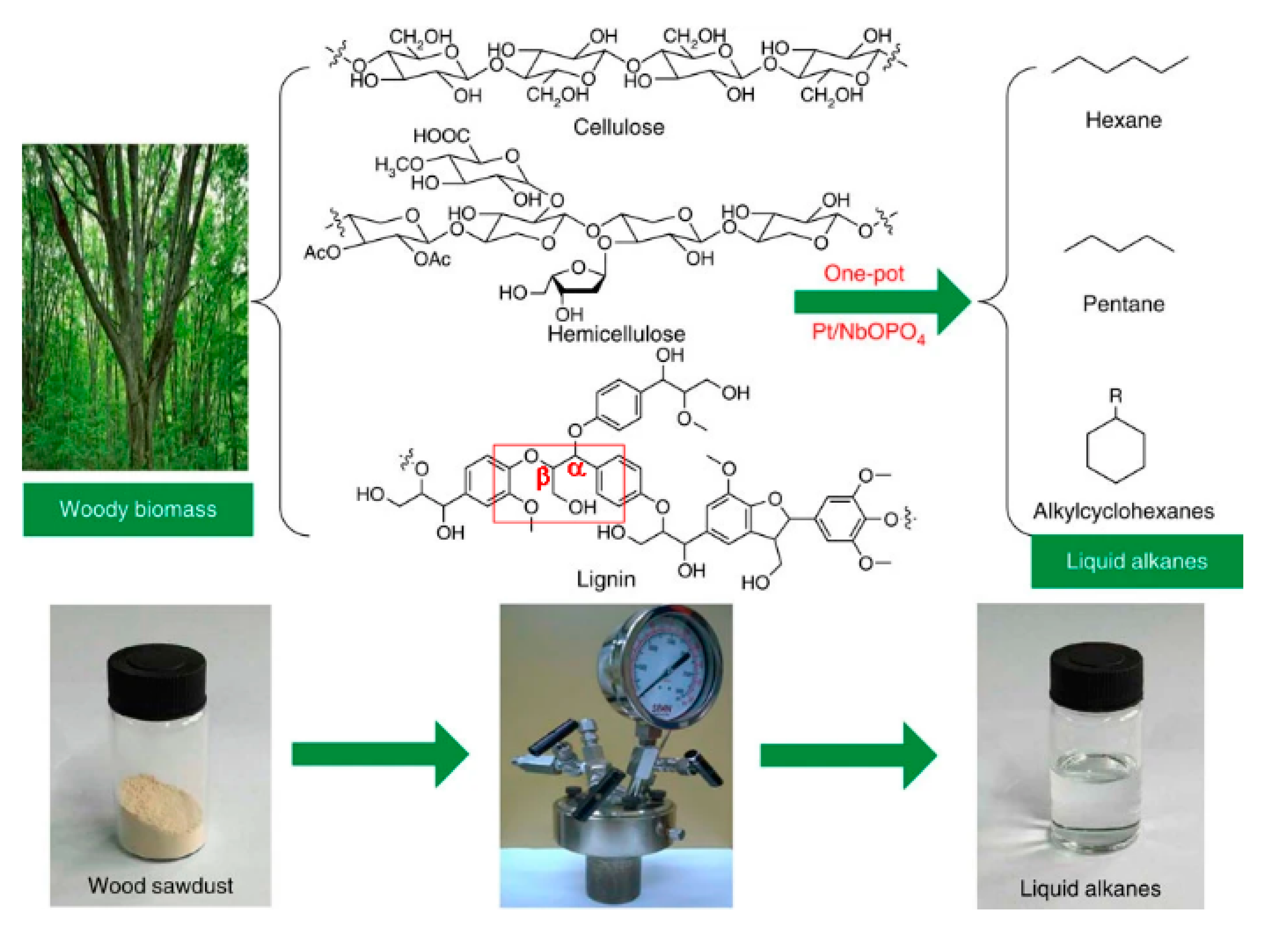
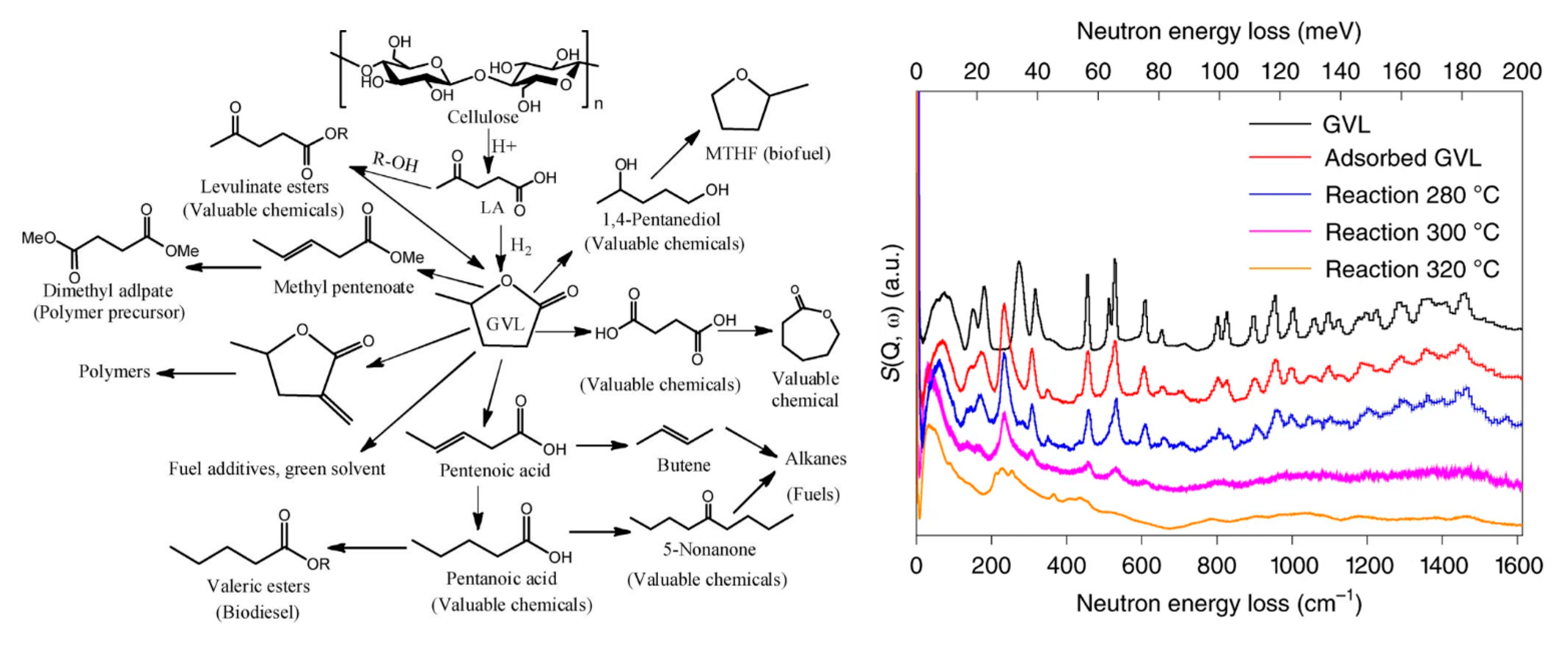
3.6. Biomass
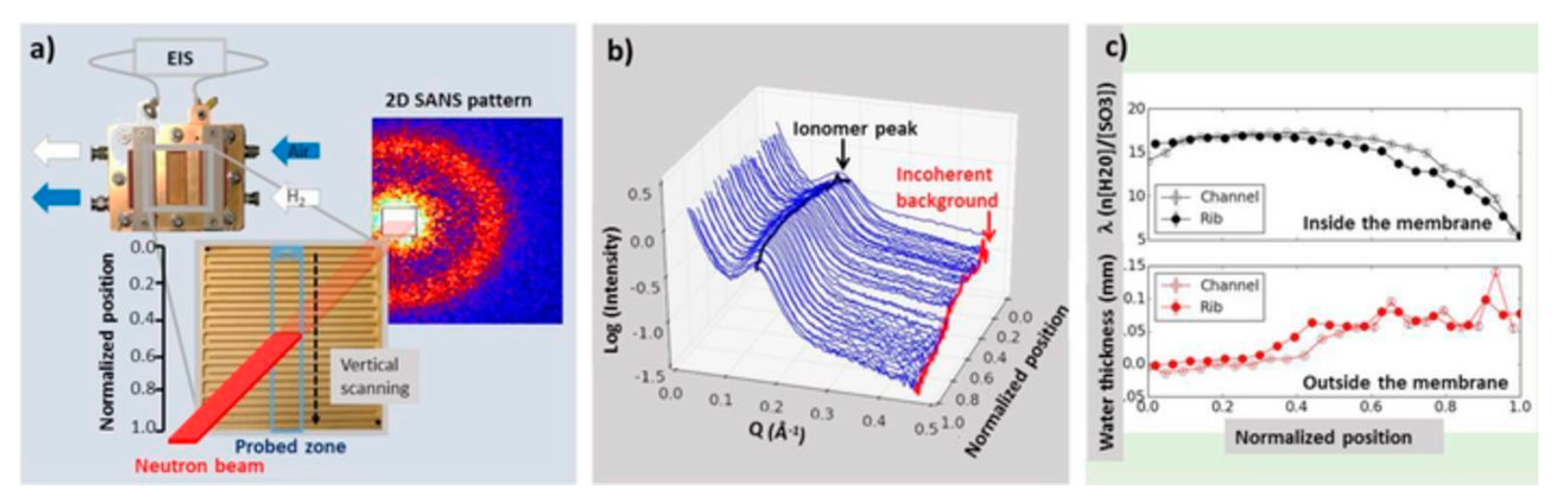
3.7. Fuel Cells
3.8. CO2 Capture and Utilization
3.9. Neutron Imaging
4. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- UK Government. Available online: https://www.gov.uk/government/news/uk-becomes-first-major-economy-to-pass-net-zero-emissions-law (accessed on 7 March 2021).
- International Energy Agency. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 23 May 2021).
- The Institute for Government. Available online: https://www.instituteforgovernment.org.uk/explainers/net-zero-target (accessed on 7 March 2021).
- The Royal Society of Chemistry. Available online: https://0-www-rsc-org.brum.beds.ac.uk/globalassets/22-new-perspectives/sustainability/rsc-chemicals-strategy-policy-2020.pdf (accessed on 7 March 2021).
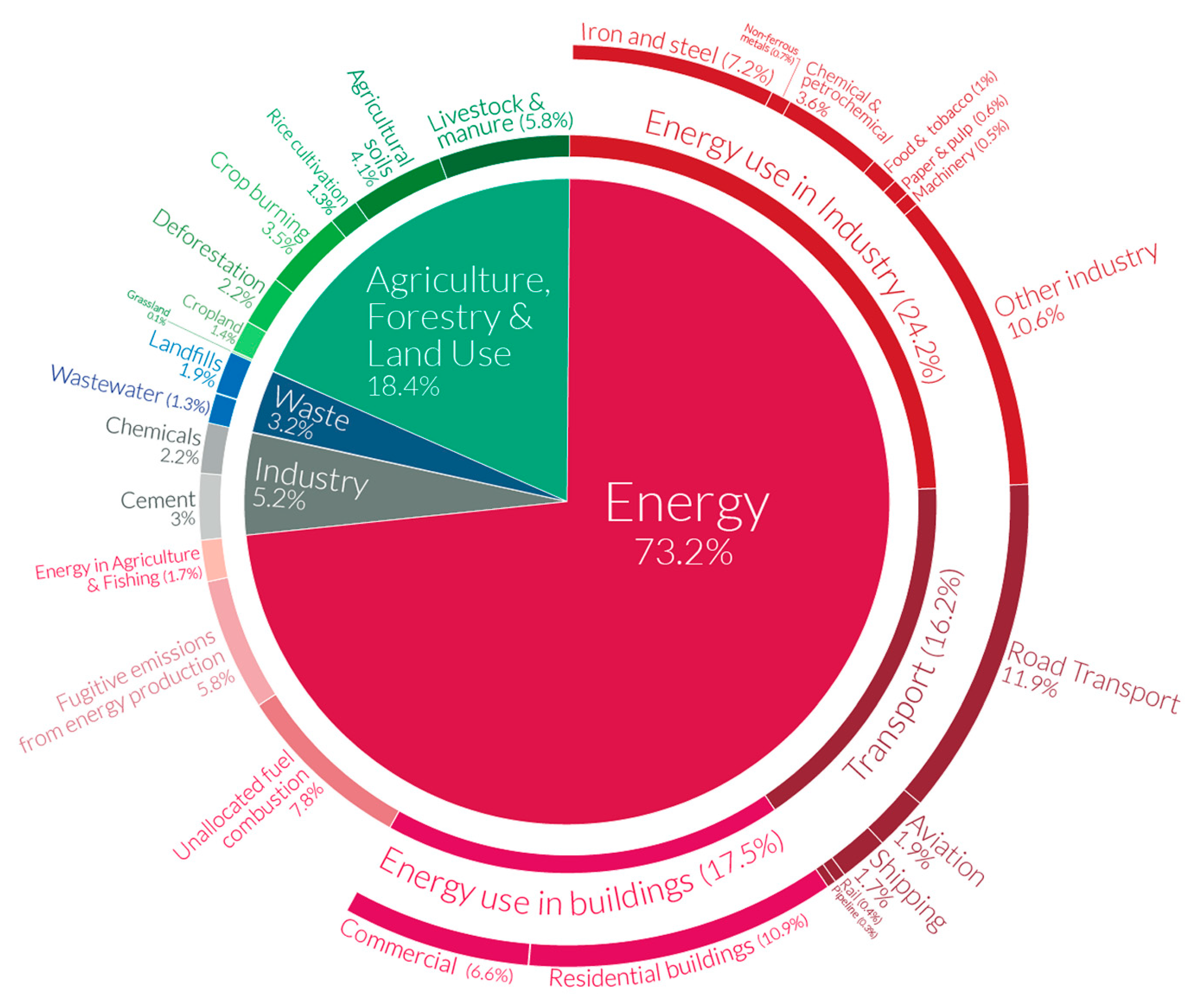
- Ritchie, H.; Roser, R. Emissions by Sector. Our World in Data. Available online: https://ourworldindata.org/emissions-by-sector (accessed on 7 March 2021).
- Catlow, C.R.A.; Davidson, M.; Hardacre, C.; Hutchings, G.J. Catalysis making the world a better place. Philos. Trans. R. Soc. A 2016, 374, 20150089. [Google Scholar] [CrossRef] [PubMed]
- Niemantsverdriet, J.W. Spectroscopy in Catalysis: An Introduction, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Squires, G.L. Introduction to the Theory of Thermal Neutron Scattering; Dover Publications: Mineola, NY, USA, 1978. [Google Scholar]
- Fernandez-Alonso, F.; Price, D.L. (Eds.) Neutron Scattering—Fundamentals, Experimental Methods in the Physical Sciences; Academic Press: Amsterdam, The Netherlands, 2013; Volume 44. [Google Scholar]
- Willis, B.T.M.; Carlile, C.J. Experimental Neutron Scattering; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Fernandez-Alonso, F.; Price, D.L. (Eds.) Neutron Scattering—Applications in Biology, Chemistry and Materials Science, Experimental Methods in the Physical Sciences; Academic Press: Amsterdam, The Netherlands, 2017; Volume 49. [Google Scholar]
- Mitchell, P.C.H.; Parker, S.F.; Ramirez-Cuesta, A.J.; Tomkinson, J. Vibrational Spectroscopy with Neutrons, with Applications in Chemistry, Biology, Materials Science and Catalysis; World Scientific: Singapore, 2005. [Google Scholar]
- Lépine-Szily, A.; Descouvemont, P. Nuclear astrophysics: Nucleosynthesis in the universe. Int. J. Astrobiol. 2012, 11, 243–250. [Google Scholar] [CrossRef]
- Neutronsources.org. Available online: https://neutronsources.org/ (accessed on 7 March 2021).
- The Institut Laue-Langevin. Available online: https://www.ill.eu/ (accessed on 7 March 2021).
- The ISIS Neutron and Muon Source. Available online: https://www.isis.stfc.ac.uk/Pages/About.aspx (accessed on 7 March 2021).
- The Spallation Neutron Source. Available online: https://neutrons.ornl.gov/sns (accessed on 7 March 2021).
- The Japan Proton Accelerator Research Complex. Available online: https://snsr.jaea.go.jp/en/research/j-parc.html (accessed on 7 March 2021).
- The European Spallation Source. Available online: https://europeanspallationsource.se/about (accessed on 7 March 2021).
- Wilson, C.C. Single Crystal Neutron Diffraction from Molecular Materials; World Scientific: Singapore, 2000. [Google Scholar]
- Kisi, E.H.; Howard, C.J. Applications of Neutron Powder Diffraction; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Anderson, I.S.; McGreevy, R.L.; Bilheux, H.Z. (Eds.) Neutron Imaging and Applications. A Reference for the Imaging Community; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Hempelmann, R. Quasielastic Neutron Scattering and Solid State Diffusion; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Andreani, C.; Krzystyniak, M.; Romanelli, G.; Senesi, R.; Fernandez-Alonso, F. Electron-volt neutron spectroscopy: Beyond fundamental systems. Adv. Phys. 2017, 66, 1–73. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Bhaduri, S.; Mukesh, D. Homogeneous Catalysis: Mechanisms and Industrial Applications, 2nd ed.; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Husain, Q.; Ullah, M.F. (Eds.) Biocatalysis; Springer: Basel, Switzerland, 2019. [Google Scholar]
- Shao, M. (Ed.) Electrocatalysis; Springer: Basel, Switzerland, 2020. [Google Scholar]
- International Energy Agency. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 23 May 2021).
- Boretti, A. Technology readiness level of solar thermochemical splitting cycles. ACS Energy Lett. 2021, 6, 1170–1174. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Ďurovič, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the hydrogen evolution reaction in alkaline and neutral media. A comparative review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Murkin, C.; Brightling, J. Eighty years of steam reforming. Johns. Matthey Technol. Rev. 2016, 60, 263–269. [Google Scholar] [CrossRef]
- McFarlane, A.R.; Silverwood, I.P.; Norris, E.L.; Ormerod, R.M.; Frost, C.D.; Parker, S.F.; Lennon, D. The application of inelastic neutron scattering to investigate the steam reforming of methane over an alumina-supported nickel catalyst. Chem. Phys. 2013, 427, 54–60. [Google Scholar] [CrossRef]
- Silverwood, I.P.; Hamilton, N.G.; Laycock, C.J.; Staniforth, J.Z.; Ormerod, R.M.; Frost, C.D.; Parker, S.F.; Lennon, D. Quantification of surface species present on a nickel/alumina methane reforming catalyst. Phys. Chem. Chem. Phys. 2010, 12, 3102–3107. [Google Scholar] [CrossRef]
- Silverwood, I.P.; Hamilton, N.G.; Staniforth, J.Z.; Laycock, C.J.; Parker, S.F.; Ormerod, M.; Lennon, D. Persistent species formed during the carbon dioxide reforming of methane over a nickel-alumina catalyst. Catal. Today 2010, 155, 319–325. [Google Scholar] [CrossRef]
- Silverwood, I.P.; Hamilton, N.G.; McFarlane, A.R.; Kapitán, J.; Hecht, L.; Norris, E.L.; Ormerod, R.M.; Frost, C.D.; Parker, S.F.; Lennon, D. Application of inelastic neutron scattering to studies of CO2 reforming of methane over alumina-supported nickel and gold-doped nickel catalysts. Phys. Chem. Chem. Phys. 2012, 14, 15214–15225. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, A.R.; Silverwood, I.P.; Warringham, R.; Norris, E.L.; Ormerod, R.M.; Frost, C.D.; Parker, S.F.; Lennon, D. The application of inelastic neutron scattering to investigate the ‘dry’ reforming of methane over an alumina-supported nickel catalyst operating under conditions where filamentous carbon formation is prevalent. RSC Adv. 2013, 3, 16577–16589. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Gerdts, R.; Parker, S.F.; Chi, L.; Zhao, Y.; Hill, M.; Jones, M.O.; Jiang, Z. In-depth understanding of the bimetallic effects and coked carbon species on an active bimetallic Ni(Co)/Al2O3 dry reforming catalyst. Phys. Chem. Chem. Phys. 2016, 18, 17311–17319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverwood, I.P.; Hamilton, N.G.; McFarlane, A.R.; Ormerod, R.M.; Guidi, T.; Bones, J.; Dudman, M.P.; Goodway, C.M.; Kibble, M.; Parker, S.F.; et al. Experimental arrangements suitable for the acquisition of inelastic neutron scattering spectra of heterogeneous catalysts. Rev. Sci. Inst. 2011, 82, 034101. [Google Scholar] [CrossRef]
- Hooper, C.W. Ammonia synthesis: Commercial practice. In Catalytic Ammonia Synthesis; Jennings, J.R., Ed.; Springer: Boston, MA, USA, 1991. [Google Scholar] [CrossRef]
- Kandemir, T.; Girgsdies, F.; Kasatkin, I.; Kunkes, E.; Liss, K.-D.; Peterson, V.K.; Schlögl, R.; Behrens, M. Heterogeneous Catalysis under pressure—In-situ neutron diffraction under industrial conditions. J. Phys. Conf. Ser. 2012, 340, 012053. [Google Scholar] [CrossRef] [Green Version]
- Kandemir, T.; Schuster, M.E.; Senyshyn, A.; Behrens, M.; Schlögl, R. The Haber–Bosch process revisited: On the real structure and stability of “ammonia iron” under working conditions. Angew. Chem. Int. Ed. 2013, 52, 12723–12726. [Google Scholar] [CrossRef]
- Wood, T.J.; Makepeace, J.W.; David, W.I.F. Neutron diffraction and gravimetric study of the iron nitriding reaction under ammonia decomposition conditions. Phys. Chem. Chem. Phys. 2017, 19, 27859–27865. [Google Scholar] [CrossRef]
- Von de Loosdrecht, J.; Botes, F.G.; Ciobica, I.M.; Ferreira, A.; Gibson, P.; Moodley, D.J.; Saib, A.M.; Visagie, J.L.; Westrate, C.J.; Niemantsverdriet, J.W. Fischer–Tropsch synthesis: Catalysts and chemistry. In Comprehensive Inorganic Chemistry II; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Oxford, UK, 2013; Volume 7, pp. 525–557. [Google Scholar] [CrossRef]
- Torres, G.H.M.; de Jong, K.P. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Paalanen, P.P.; Weckhuysen, B.M. Carbon pathways, sodium-sulphur promotion and identification of iron carbides in iron-based Fischer-Tropsch synthesis. ChemCatChem 2020, 12, 4202–4223. [Google Scholar] [CrossRef]
- Hamilton, N.G.; Silverwood, I.P.; Warringham, R.; Kapitán, J.; Hecht, L.; Webb, P.B.; Tooze, R.P.; Parker, S.F.; Lennon, D. Vibrational analysis of an industrial Fe-based Fischer-Tropsch catalyst employing inelastic neutron scattering. Angew. Chem. Int. Ed. 2013, 52, 5608–5611. [Google Scholar] [CrossRef]
- Hamilton, N.G.; Warringham, R.; Silverwood, I.P.; Kapitán, J.; Hecht, L.; Webb, P.B.; Tooze, R.P.; Zhou, W.; Frost, C.D.; Parker, S.F.; et al. The application of inelastic neutron scattering to investigate CO hydrogenation over an iron Fischer-Tropsch synthesis catalyst. J. Catal. 2014, 312, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Warringham, R.; Hamilton, N.G.; Silverwood, I.P.; How, C.; Webb, P.B.; Tooze, R.P.; Zhou, W.; Frost, C.D.; Parker, S.F.; Lennon, D. The application of inelastic neutron scattering to investigate a hydrogen pre-treatment stage of an iron Fischer-Tropsch catalyst. Appl. Catal. A Gen. 2015, 489, 209–217. [Google Scholar] [CrossRef]
- Warringham, R.; McFarlane, A.R.; MacLaren, D.A.; Webb, P.B.; Tooze, R.P.; Taylor, J.; Ewings, R.A.; Parker, S.F.; Lennon, D. The application of inelastic neutron scattering to explore the significance of a magnetic transition in an iron based Fischer-Tropsch catalyst that is active for the hydrogenation of CO. J. Chem. Phys. 2015, 143, 174703. [Google Scholar] [CrossRef] [PubMed]
- Warringham, R.; Davidson, A.L.; Webb, P.B.; Tooze, R.P.; Parker, S.F.; Lennon, D. Examining the temporal behavior of the hydrocarbonaceous overlayer on an iron based Fischer-Tropsch catalyst. RSC Adv. 2019, 9, 2608–2617. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.L.; Webb, P.B.; Parker, S.F.; Lennon, D. Hydrogen partitioning as a function of time-on-stream for an un-promoted iron-based Fischer-Tropsch synthesis catalyst applied to CO hydrogenation. Ind. Eng. Chem. Res. 2019, 59, 52–60. [Google Scholar] [CrossRef]
- Warringham, R.; Davidson, A.L.; Webb, P.B.; Tooze, R.P.; Parker, S.F.; Lennon, D. Perspectives on the effect of sulfur on the hydrocarbonaceous overlayer on iron Fischer-Tropsch catalysts. Catal. Today 2020, 339, 32–39. [Google Scholar] [CrossRef]
- Davidson, A.L.; Gibson, E.K.; Cibin, G.; Vanrensburg, H.; Parker, S.F.; Webb, P.B.; Lennon, D. The application of inelastic neutron scattering to investigate iron-based Fischer-Tropsch to olefins catalysis. J. Catal. 2020, 393, 197–208. [Google Scholar] [CrossRef]
- Davidson, A.L.; Webb, P.B.; Parker, S.F.; Lennon, D. An inelastic neutron scattering investigation of the temporal behaviour of the hydrocarbonaceous overlayer of a prototype Fischer-Tropsch to olefins catalyst. Top. Catal. 2021. [Google Scholar] [CrossRef]
- Davidson, A.L.; Lennon, D.; Webb, P.B.; Albers, P.W.; Berweiler, M.; Poss, R.; Roos, M.; Reinsdorf, A.; Wolf, D.; Parker, S.F. The characterisation of hydrogen on nickel and cobalt catalysts. Top. Catal. 2021. [Google Scholar] [CrossRef]
- The Methanol Institute. Available online: https://www.methanol.org/methanol-price-supply-demand/ (accessed on 9 April 2021).
- Waugh, K.C. Methanol synthesis. Catal. Lett. 2012, 142, 1153–1166. [Google Scholar] [CrossRef]
- Trunov, V.A.; Sokolov, A.E.; Lebedev, V.T.; Smirnov, O.P.; Kurbakov, A.I.; van den Heuvel, J.; Batyrev, E.; Yurieva, T.M.; Plyasova, L.M.; Török, G. Detection of hydrogen-copper clustering in Zn1−x CuxO compounds using neutron scattering methods. Phys. Solid State 2006, 48, 1291–1297. [Google Scholar] [CrossRef]
- Trunov, V.A.; Lebedev, V.T.; Sokolov, A.E.; Grushko, Y.S.; Török, G.; van den Heuvel, J.; Batyrev, E.; Yurieva, T.M.; Plyasova, L.M. Investigation of the hydrogen capacity of composites based on ZnOCu. Crystallog. Rep. 2007, 52, 474–478. [Google Scholar] [CrossRef]
- Kandemir, T.; Wallacher, D.; Hansen, T.C.; Liss, K.-D.; d’Alnoncourt, R.N.; Schlögl, R.; Behrens, M. In situ neutron diffraction under high pressure—Providing an insight into working catalysts. Nucl. Instrum. Methods Phys. Res. Sect. A 2012, 673, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Kandemir, T.; Girgsdies, F.; Hansen, T.C.; Liss, K.-D.; Kasatkin, I.; Kunkes, E.L.; Wowsnick, G.; Jacobsen, N.; Schlögl, R.; Behrens, M. In situ study of catalytic processes: Neutron diffraction of a methanol synthesis catalyst at industrially relevant pressure. Angew. Chem. Int. Ed. 2013, 52, 5166–5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandemir, T.; Kasatkin, I.; Girgsdies, F.; Zander, S.; Kühl, S.; Tovar, M.; Schlögl, R.; Behrens, M. Microstructural and defect analysis of metal nanoparticles in functional catalysts by diffraction and electron microscopy: The Cu/ZnO catalyst for methanol synthesis. Top. Catal. 2014, 57, 188–206. [Google Scholar] [CrossRef] [Green Version]
- Khassin, A.A.; Jobic, H.; Filonenko, G.A.; Dokuchits, E.V.; Khasin, A.V.; Minyukova, T.P.; Shtertser, N.V.; Plyasova, L.M.; Yurieva, T.M. Interaction of hydrogen with Cu–Zn mixed oxide model methanol synthesis catalyst. J. Mol. Catal. A 2013, 373, 151–160. [Google Scholar] [CrossRef]
- Kandemir, T.; Friedrich, M.; Parker, S.F.; Studt, F.; Lennon, D.; Schlögl, R.; Behrens, M. Different routes to methanol: Inelastic neutron scattering spectroscopy of adsorbates on supported copper catalysts. Phys. Chem. Chem. Phys. 2016, 18, 17253–17258. [Google Scholar] [CrossRef] [Green Version]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Haw, J.F.; Song, W.; Marcus, D.M.; Nicholas, J.B. The mechanism of methanol to hydrocarbon catalysis. Acc. Chem. Res. 2003, 36, 317–326. [Google Scholar] [CrossRef]
- Gogate, M.R. Methanol-to-olefins process technology: Current status and future prospects. Pet. Sci. Technol. 2019, 37, 559–565. [Google Scholar] [CrossRef]
- Howe, R.F.; McGregor, J.; Parker, S.F.; Collier, P.; Lennon, D. Application of inelastic neutron scattering to the methanol-to-gasoline reaction over a ZSM-5 catalyst. Catal. Lett. 2016, 46, 1242–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwardiyanto; Howe, R.F.; Gibson, E.K.; Catlow, C.R.A.; Hameed, A.; McGregor, J.; Collier, P.; Parker, S.F.; Lennon, D. An assessment of hydrocarbon species in the methanol-to-hydrocarbon reaction over a ZSM-5 catalyst. Faraday Discuss. 2017, 197, 447–471. [Google Scholar] [CrossRef] [Green Version]
- Matam, S.K.; O’Malley, A.J.; Catlow, C.R.A.; Suwardiyanto; Collier, P.; Hawkins, A.; Zachariou, A.; Lennon, D.; Silverwood, I.; Parker, S.F.; et al. The effects of MTG catalysis on methanol mobility in ZSM-5. Cat. Sci. Technol. 2018, 8, 3304–3312. [Google Scholar] [CrossRef] [Green Version]
- Zachariou, A.; Hawkins, A.P.; Suwardiyanto; Collier, P.; Barrow, N.; Howe, R.F.; Parker, S.F.; Lennon, D. New spectroscopic insight into the deactivation of a ZSM-5 methanol-to-hydrocarbons catalyst. ChemCatChem 2021. [Google Scholar] [CrossRef]
- Lin, L.; Fan, M.; Sheveleva, A.M.; Han, X.; Tang, Z.; Carter, J.H.; da Silva, I.; Parlett, C.M.A.; Tuna, F.; McInnes, E.J.L.; et al. Control of zeolite microenvironment for propene synthesis from methanol. Nat. Commun. 2021, 12, 822. [Google Scholar] [CrossRef]
- Zachariou, A.; Hawkins, A.P.; Lennon, D.; Parker, S.F.; Suwardiyanto; Matam, S.K.; Catlow, C.R.A.; Collier, P.; Hameed, A.; McGregor, J.; et al. Investigation of ZSM-5 catalysts for dimethylether conversion using inelastic neutron scattering. Appl. Catal. A Gen. 2019, 569, 1–7. [Google Scholar] [CrossRef]
- Zachariou, A.; Hawkins, A.P.; Collier, P.; Howe, R.F.; Parker, S.F.; Lennon, D. The effect of co-feeding methyl acetate on the H-ZSM5 catalysed methanol-to-hydrocarbons reaction. Top. Catal. 2020, 63, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Zachariou, A.; Hawkins, A.P.; Collier, P.; Howe, R.F.; Lennon, D.; Parker, S.F. The methyl torsion in unsaturated compounds. ACS Omega 2020, 5, 2755–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; Chen, Z.; Shao, Y.; Gong, X.; Wang, H.; Liu, X.; Parker, S.F.; Han, X.; Yang, S.; Wang, Y. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun. 2016, 7, 11162. [Google Scholar] [CrossRef] [PubMed]
- Adilina, I.B.; Rinaldi, N.; Simanungkalit, S.; Aulia, F.; Oemry, F.; Stenning, G.; Silverwood, I.P.; Parker, S.F. Hydrodeoxygenation of guaiacol as a bio-oil model compound over pillared clay-supported nickel–molybdenum catalysts. J. Phys. Chem. C 2019, 123, 21429–21439. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef]
- Dong, L.; Shao, Y.; Han, X.; Liu, X.; Xia, Q.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, Y. Comparison of two multifunctional catalysts [M/Nb2O5 (M = Pd, Pt)] for one-pot hydrodeoxygenation of lignin. Cat. Sci. Technol. 2018, 8, 6129–6136. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Lin, L.; Han, X.; Si, X.; Liu, X.; Guo, Y.; Lu, F.; Rudić, S.; Parker, S.F.; Yang, S.; et al. Breaking the limit of lignin monomer production via cleavage of interunit carbon–carbon linkages. Chem 2019, 6, 1521–1536. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic reactions of gamma-valerolactone: A platform to fuels and value-added chemicals. Appl. Catal. B Environ. 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Lin, L.; Sheveleva, A.M.; da Silva, I.; Parlett, C.M.A.; Tang, Z.; Liu, Y.; Fan, M.; Han, X.; Carter, J.H.; Tuna, F.; et al. Quantitative production of butenes from biomass-derived γ-valerolactone catalysed by hetero-atomic MFI zeolite. Nat. Mater. 2020, 19, 86–93. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Loh, K.S. A review on synthesis and characterization of solid acid materials for fuel cell applications. J. Power Sources 2016, 322, 77–92. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef] [Green Version]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Asada, H.; Toya, T. Study of hydrogen adsorbed on platinum by neutron inelastic scattering spectroscopy. J. Chem. Phys. 1975, 63, 4078–4079. [Google Scholar] [CrossRef]
- Rush, J.J.; Cavanagh, R.R.; Kelley, R.D. Neutron scattering from adsorbates on platinum black. J. Vac. Sci. Technol. A 1983, 1, 1245–1246. [Google Scholar] [CrossRef]
- Renouprez, A.J.; Jobic, H. Neutron scattering study of hydrogen adsorption on platinum catalysts. J. Catal. 1988, 113, 509–516. [Google Scholar] [CrossRef]
- Albers, P.; Auer, E.; Ruth, K.; Parker, S.F. Inelastic neutron scattering investigation of the nature of surface sites occupied by hydrogen on highly dispersed platinum on commercial carbon black supports. J. Catal. 2000, 196, 174–179. [Google Scholar] [CrossRef]
- Albers, P.W.; Lopez, M.; Sextl, G.; Jeske, G.; Parker, S.F. Inelastic neutron scattering investigation on the site occupation of atomic hydrogen on platinum particles of different size. J. Catal. 2004, 223, 44–53. [Google Scholar] [CrossRef]
- Carosso, M.; Vottero, E.; Lazzarini, A.; Morandi, S.; Manzoli, M.; Lomachenko, K.A.; Jiménez-Ruiz, M.; Pellegrini, R.; Lamberti, C.; Piovano, A.; et al. Dynamics of reactive species and reactant-induced reconstruction of Pt clusters in Pt/Al2O3 catalysts. ACS Catal. 2019, 9, 7124–7136. [Google Scholar] [CrossRef]
- Parker, S.F.; Mukhopadhyay, S.; Jiménez-Ruiz, M.; Albers, P.W. Adsorbed states of hydrogen on platinum: A new perspective. Chem. Eur. J. 2019, 25, 6496–6499. [Google Scholar] [CrossRef] [Green Version]
- Parker, S.F.; Shah, S. Characterisation of hydration water in Nafion membrane. RSC Adv. 2012, 11, 9381–9385. [Google Scholar] [CrossRef]
- Perrin, J.-C.; Lyonnard, S.; Volino, F. Quasielastic neutron scattering study of water dynamics in hydrated Nafion membranes. J. Phys. Chem. C 2007, 111, 3393–3404. [Google Scholar] [CrossRef]
- Martinez, N.; Arnaud, M.A.; Berrod, Q.; Frick, B.; Ollivier, J.; Porcar, L. Multiscale water dynamics in a fuel cell by operando quasi elastic neutron scattering. J. Phys. Chem. C 2018, 122, 1103–1108. [Google Scholar] [CrossRef]
- Yamada, T.; Tominaga, T. In situ quasi-elastic neutron scattering of Nafion membrane with water-vapor pressure control system. JPS Conf. Proc. 2021, 33, 011085. [Google Scholar] [CrossRef]
- Kim, M.-H.; Glinka, C.J.; Grot, S.A.; Grot, W.G. SANS study of the effects of water vapor sorption on the nanoscale structure of perfluorinated sulfonic acid (NAFION) membranes. Macromolecules 2006, 39, 4775–4787. [Google Scholar] [CrossRef] [Green Version]
- Morin, A.; Gebel, G.; Porcar, L.; Peng, Z.; Martinez, N.; Guillermo, A.; Lyonnard, S. Quantitative multi-scale operando diagnosis of water localization inside a fuel cell. J. Electrochem. Soc. 2017, 164, F9–F21. [Google Scholar] [CrossRef]
- Ishikawa, A.; Maekawa, H.; Yamamura, T.; Kawakita, Y.; Shibata, K.; Kawai, M. Proton dynamics of CsH2PO4 studied by quasi-elastic neutron scattering and PFG-NMR. Solid State Ionics 2008, 149, 2345–2349. [Google Scholar] [CrossRef]
- Fillaux, F.; Marchon, B.; Novak, A.; Tomkinson, J. Proton dynamics in the hydrogen bond, inelastic neutron scattering by single crystals of CsH2PO4 at 20 K. Chem. Phys. 1989, 130, 257–270. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Carlile, C.J.; Shuvalov, L.A. The diffusion of protons in the superionic conductor CsHSO4 by quasielastic neutron scattering. J. Phys. Condens. Matter 1992, 4, 389–398. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Adams, M.A.; Kolesnikov, A.I.; Shuvalov, L.A. Lattice dynamics and effects of anharmonicity in different phases of caesium hydrogen sulphate. J. Phys. Condens. Matter 1994, 6, 5823–5832. [Google Scholar] [CrossRef]
- Krzystyniak, M.; Drużbicki, K.; Fernandez-Alonso, F. Nuclear dynamics in the metastable phase of the solid acid caesium hydrogen sulfate. Phys. Chem. Chem. Phys. 2015, 17, 31287–31296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, S.F.; Cavaye, H.; Callear, S.K. Structure and dynamics of the superprotonic conductor caesium hydrogen sulfate, CsHSO4. Molecules 2020, 25, 1271. [Google Scholar] [CrossRef] [Green Version]
- Kreuer, K.-D. On solids with liquid-like properties and the challenge to develop new proton-conducting separator materials for intermediate temperature fuel cells. ChemPhysChem 2002, 3, 771–775. [Google Scholar] [CrossRef]
- Karlsson, M. Perspectives of neutron scattering on proton conducting. Dalton Trans. 2013, 42, 317–329. [Google Scholar] [CrossRef]
- Kendrick, E.; Knight, K.S.; Islam, M.S.; Slater, P.R. Structural studies of the proton conducting perovskite ‘La0.6Ba0.4ScO2.8’. Solid State Ionics 2007, 178, 943–949. [Google Scholar] [CrossRef]
- Mather, G.C.; Heras-Juaristi, G.; Ritter, C.; Fuentes, R.O.; Chinelatto, A.L.; Perez-Coll, D.; Amador, U. Phase transitions, chemical expansion, and deuteron sites in the BaZr0.7Ce0.2Y0.1O3−δ proton conductor. Chem. Mater. 2016, 218, 4292–4299. [Google Scholar] [CrossRef]
- Kinyanjui, F.G.; Norberg, S.T.; Ahmed, I.; Eriksson, S.G.; Hull, S. In-situ conductivity and hydration studies of proton conductors using neutron powder diffraction. Solid State Ionics 2012, 225, 312–316. [Google Scholar] [CrossRef]
- Noferini, D.; Koza, M.M.; Nilsen, G.J.; Karlsson, M. Study of the hydration level in proton conducting oxides using neutron diffraction with polarization analysis. Solid State Ionics 2018, 324, 163–167. [Google Scholar] [CrossRef]
- Karlsson, M. Proton dynamics in oxides: Insight into the mechanics of proton conduction from quasielastic neutron scattering. Phys. Chem. Chem. Phys. 2015, 17, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Noferini, D.; Koza, M.M.; Rahman, S.M.H.; Evenson, Z.; Nilsen, G.J.; Eriksson, S.; Wildes, A.R.; Karlsson, M. Role of the doping level in localized proton motions in acceptor-doped barium zirconate proton conductors. Phys. Chem. Chem. Phys. 2018, 20, 13697–13704. [Google Scholar] [CrossRef]
- Karlsson, M.; Matic, A.; Parker, S.F.; Ahmed, I.; Börjesson, L.; Eriksson, S. O-H wag vibrations in hydrated BaInxZr1-xO3-x/2. Phys. Rev. B 2008, 77, 104302. [Google Scholar] [CrossRef]
- Bielecki, J.; Parker, S.F.; Ekanayake, D.; Börjesson, L.; Karlsson, M. Short-range structure and phonon assignment of the brownmillerite-type oxide Ba2In2O5 and its hydrated proton-conducting form BaInO3H. J. Mater. Chem. A 2014, 2, 16915–16924. [Google Scholar] [CrossRef]
- Perrichon, A.; Jiménez-Ruiz, M.; Mazzei, L.; Rahman, S.M.H.; Karlsson, M. Local structure and vibrational dynamics of proton conducting Ba2In2O5(H2O)x. J. Mater. Chem. A 2019, 7, 17626–17636. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, L.; Perrichon, A.; Mancini, A.; Wahnström, G.; Malavasi, L.; Parker, S.F.; Börjesson, L.; Karlsson, M. Local structure and vibrational dynamics in indium-doped barium zirconate. J. Mater. Chem. A 2019, 7, 7360–7372. [Google Scholar] [CrossRef] [Green Version]
- Perrichon, A.; Torino, N.; Granhed, E.J.; Lin, Y.-C.; Parker, S.F.; Jiménez-Ruiz, M.; Karlsson, M.; Henry, P. Local coordination environments and vibrational dynamics of protons in hexagonal and cubic Sc-doped BaTiO3 proton-conducting oxides. J. Phys. Chem. C 2020, 124, 8643–8651. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, V.; Magrez, A.; Caldes, M.; Joubert, O.; Taulelle, F.; Rodriguez-Carvajal, J.; Piffard, Y.; Brohan, L. Characterization of perovskite systems derived from Ba2In2O5? Part II: The proton compounds Ba2In2(1-x)Ti2xO4+2x(OH)y [0 ≤ x ≤ 1; y ≤ 2(1-x)]. Solid State Ionics 2004, 170, 25–32. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Ren, F.; An, K. Visualizing the structural evolution of LSM/xYSZ Composite cathodes for SOFC by in-situ neutron diffraction. Sci. Rep. 2014, 4, 5179. [Google Scholar] [CrossRef] [Green Version]
- Hull, S. In-situ neutron diffraction experiments. In Electro-Chemo-Mechanics of Solids; Bishop, S., Perry, N., Marrocchelli, D., Sheldon, B., Eds.; Springer: Cham, Switzerland, 2017; pp. 66–101. [Google Scholar] [CrossRef]
- Sarno, C.; Yang, T.; Di Bartolomeo, E.; Huq, A.; Huang, H.; McIntosh, S. Oxygen vacancy localization and anisotropic oxygen anion transport in Sr1-xYxCoO3-δ (x = 0.1, 0.2) under solid oxide fuel cell cathode conditions. Solid State Ionics 2018, 321, 34–42. [Google Scholar] [CrossRef]
- Jarvis, S.M.; Samsatli, S. Technologies and infrastructures underpinning future CO2 value chains: A comprehensive review and comparative analysis. Renew. Sustain. Energy Rev. 2018, 85, 46–68. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Dowell, N.M.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Hussein, I.A.; Al-Marri, M.J.; Mahmoud, M.; Shawabkeh, R.; Aparicio, S. CO2 enhanced gas recovery and sequestration in depleted gas reservoirs: A review. J. Pet. Sci. Eng. 2021, 196, 107685. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Experimental study on natural gas hydrate exploitation: Optimization of methane recovery, carbon dioxide storage and deposit structure preservation. J. Pet. Sci. Eng. 2019, 177, 594–601. [Google Scholar] [CrossRef]
- Gambelli, A.M. An experimental description of the double positive effect of CO2 injection in methane hydrate deposits in terms of climate change mitigation. Chem. Eng. Sci. 2021, 233, 116430. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-based CO2 capture technology development from the beginning of 2013—A review. ACS Appl. Mater. Interfaces 2015, 7, 2137–2148. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Benson, O.; da Silva, I.; Argent, S.P.; Cabot, R.; Savage, M.; Godfrey, H.G.W.; Yan, Y.; Parker, S.F.; Manuel, P.; Lennox, M.J.; et al. Amides do not always work: Observation of guest binding in an amide-functionalised porous host. J. Am. Chem. Soc. 2016, 138, 14828–14831. [Google Scholar] [CrossRef]
- Easun, T.L.; Moreau, F.; Yan, Y.; Yang, S.; Schröder, M. Structural and dynamic studies of substrate binding in porous metal—Organic frameworks. Chem. Soc. Rev. 2017, 46, 239–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, F.; da Silva, I.; Al Smail, N.H.; Easun, T.L.; Savage, M.; Godfrey, H.G.W.; Parker, S.F.; Manuel, P.; Yang, S.; Schröder, M. Unravelling exceptional acetylene and carbon dioxide adsorption within a tetra-amide functionalized metal-organic framework. Nat. Commun. 2017, 8, 14085. [Google Scholar] [CrossRef]
- Wen, H.-M.; Liao, C.; Libo, L.L.; Alsalme, A.; Alothman, Z.; Krishna, R.; Wu, H.; Zhou, W.; Hu, J.; Chen, B. A metal–organic framework with suitable pore size and dual functionalities for highly efficient post-combustion CO2 capture. J. Mater. Chem. A 2019, 7, 3128–3134. [Google Scholar] [CrossRef]
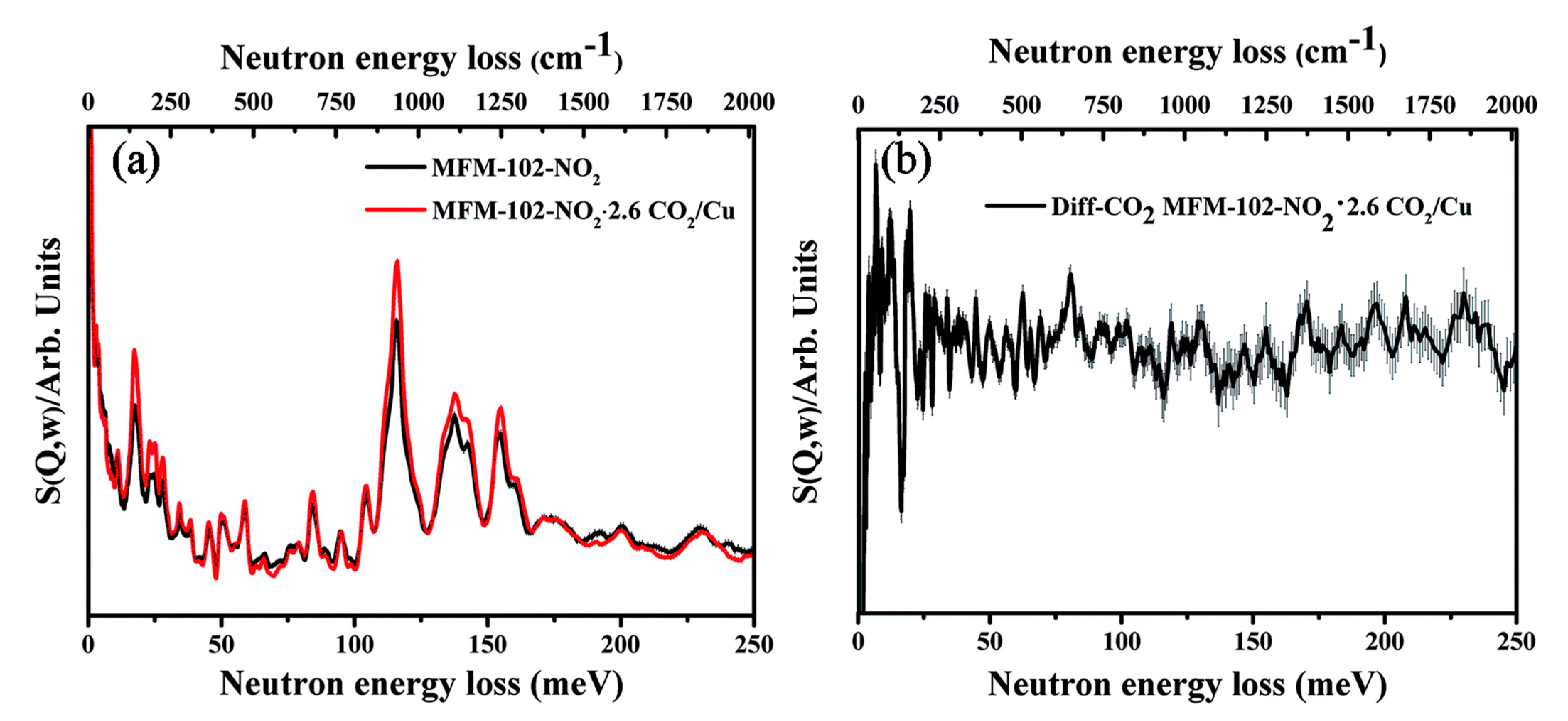
- Duong, T.D.; Sapchenko, S.A.; da Silva, I.; Godfrey, H.G.W.; Cheng, Y.; Daemen, L.L.; Manuel, P.; Frogley, M.D.; Cinque, G.; Ramirez-Cuesta, A.J.; et al. Observation of binding of carbon dioxide to nitro-decorated metal–organic frameworks. Chem. Sci. 2020, 11, 5339–5346. [Google Scholar] [CrossRef]
- Driver, J.G.; Owen, R.E.; Makanyire, T.; Lake, J.A.; McGregor, J.; Styring, P. Blue urea: Fertilizer with reduced environmental impact. Front. Energy Res. 2019. [Google Scholar] [CrossRef] [Green Version]
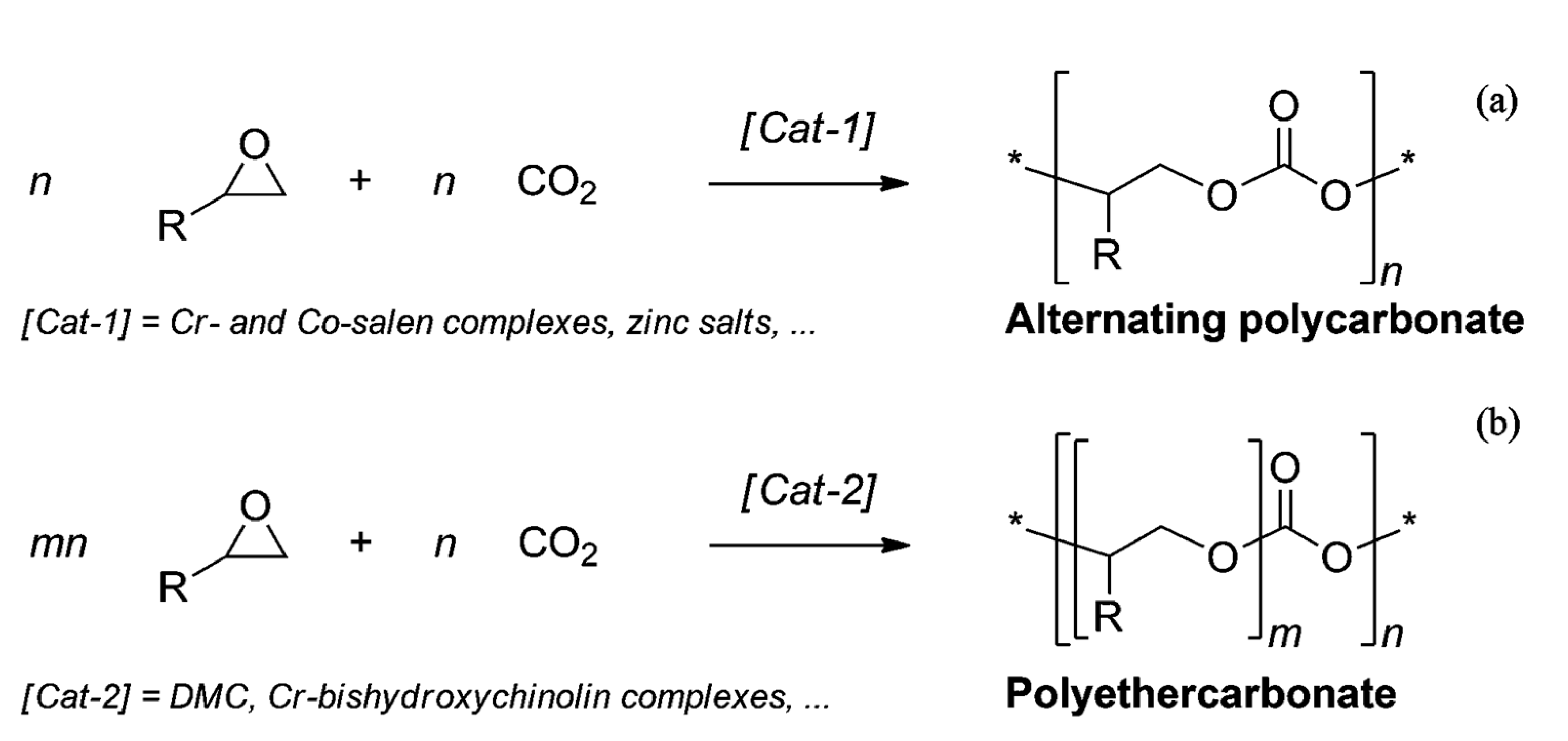
- Darensbourg, D.J.; Wilson, S.J. What’s new with CO2? Recent advances in its copolymerization with oxiranes. Green Chem. 2012, 14, 2665–2671. [Google Scholar] [CrossRef]
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A.W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef]
- Langanke, J.; Wolf, A.; Hofmann, J.; Böhm, K.; Subhani, M.A.; Müller, T.E.; Leitner, W.; Gürtler, C. Carbon dioxide (CO2) as sustainable feedstock for polyurethane production. Green Chem. 2014, 16, 1865–1870. [Google Scholar] [CrossRef]
- Jang, J.H.; Ha, J.H.; Kim, I.; Baik, J.H.; Hong, S.C. Facile room-temperature preparation of flexible polyurethane foams from carbon dioxide based poly(ether carbonate) polyols with a reduced generation of acetaldehyde. ACS Omega 2019, 4, 7944–7952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von der Assen, N.; Bardow, A. Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: Insights from an industrial case study. Green Chem. 2014, 16, 3272–3280. [Google Scholar] [CrossRef] [Green Version]
- Youngs, T.G.A.; Manyar, H.; Bowron, D.T.; Gladden, L.F.; Hardacre, C. Probing chemistry and kinetics of reactions in heterogeneous catalysts. Chem. Sci. 2013, 4, 3484–3489. [Google Scholar] [CrossRef] [Green Version]
- Falkowska, M.; Chansai, S.; Manyar, H.G.; Gladden, L.F.; Bowron, D.T.; Youngs, T.G.A.; Hardacre, C. Determination of toluene hydrogenation kinetics with neutron diffraction. Phys. Chem. Chem. Phys. 2016, 18, 17237–17243. [Google Scholar] [CrossRef] [Green Version]
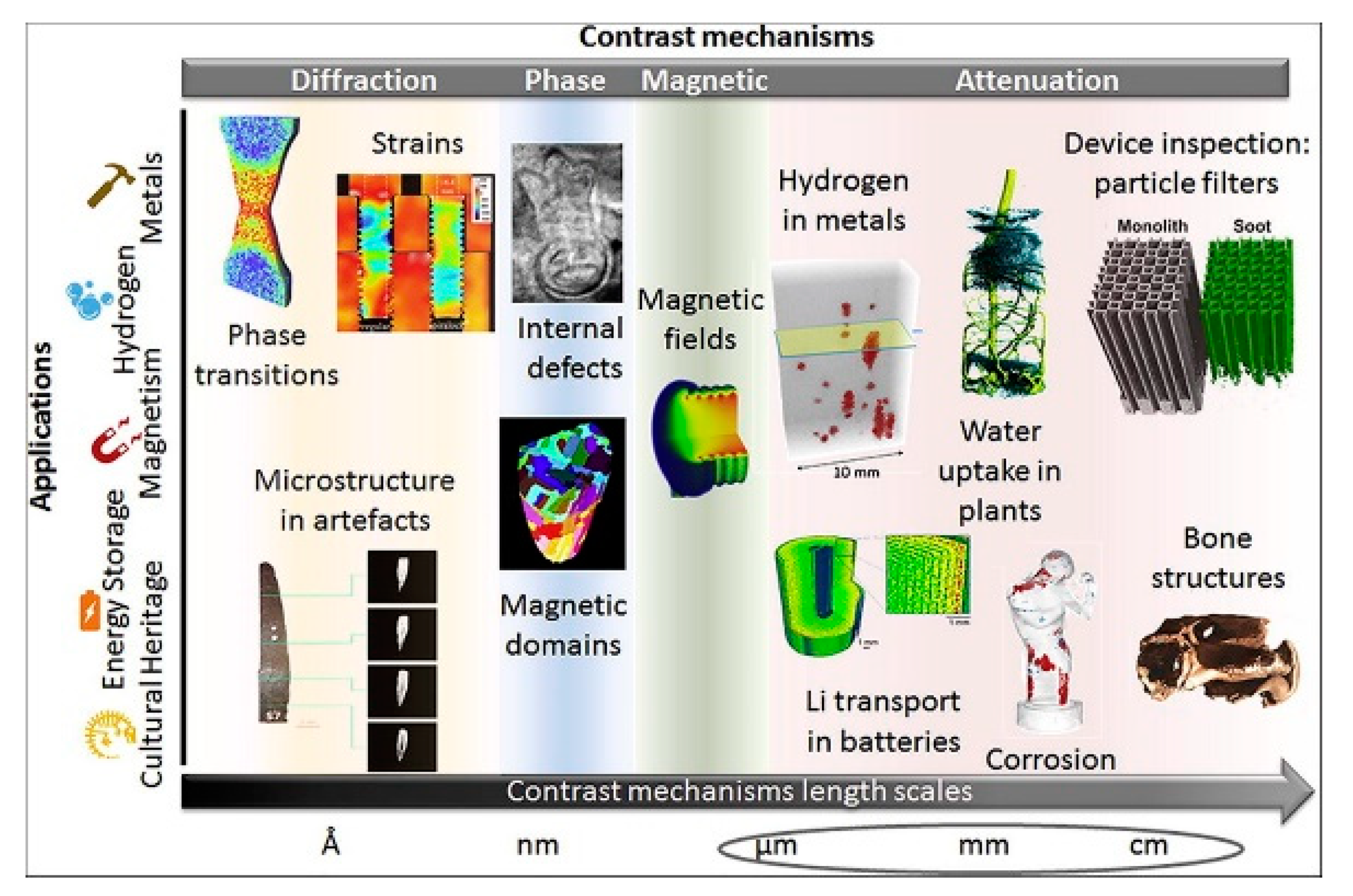
- Brenizer, J.S. A review of significant advances in neutron imaging from conception to the present. Phys. Procedia 2013, 43, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Kardjilov, N.; Manke, I.; Woracek, R.; Hilger, A.; Banhart, J. Advances in neutron imaging. Mater. Today 2018, 21, 652–672. [Google Scholar] [CrossRef]
- Siegel, J.B.; Lin, X.; Stefanopoulou, A.G.; Hussey, D.S.; Jacobson, D.L.; Gorsich, D. Neutron imaging of lithium concentration in LFP pouch cell battery. J. Electrochem. Soc. 2011, 158, A523–A529. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, Z.-G.; Li, X.; He, L.; Yu, X.; Li, H.; Wang, F. Neutron-based characterization techniques for lithium-ion battery research. Chin. Phys. B 2020, 29, 018201. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Santamaria, A.; Parka, J.W.; Lee, C.; Hwang, W. Quantification of water in hydrophobic and hydrophilic flow channels subjected to gas purging via neutron imaging. J. Power Sources 2011, 196, 9373–9381. [Google Scholar] [CrossRef]
- Manke, I.; Markötter, H.; Tötzke, C.; Kardjilov, N.; Grothausmann, R.; Dawson, M.; Hartnig, C.; Haas, S.; Thomas, D.; Hoell, A.; et al. Investigation of energy-relevant materials with synchrotron X-Rays and neutrons. Adv. Eng. Mater. 2011, 13, 712–729. [Google Scholar] [CrossRef]
- Kalyvas, C.; Kucernak, A.; Brett, D.; Hinds, G.; Atkins, S.; Brandon, N. Spatially resolved diagnostic methods for polymer electrolyte fuel cells: A review. WIREs Energy Environ. 2014, 3, 254–275. [Google Scholar] [CrossRef]
- Boillat, P.; Lehmann, E.H.; Trtik, P.; Cochet, M. Neutron imaging of fuel cells—Recent trends and future prospects. Curr. Opin. Electrochem. 2017, 5, 3–10. [Google Scholar] [CrossRef]
- Higuchi, Y.; Setoyama, D.; Isegawa, K.; Tsuchikawa, Y.; Matsumoto, Y.; Parker, J.D.; Shinohara, T.; Nagai, Y. Pulsed neutron imaging for differentiation of ice and liquid water towards fuel cell vehicle applications. Phys. Chem. Chem. Phys. 2021, 23, 1062–1071. [Google Scholar] [CrossRef]
- Ilisca, E. Ortho-para conversion of hydrogen molecules physisorbed on surfaces. Prog. Surf. Sci. 1992, 41, 217–335. [Google Scholar] [CrossRef]
- Romanelli, G.; Minniti, T.; Škoro, G.; Krzystyniak, M.; Taylor, J.; Fornalski, D.; Fernandez-Alonso, F. Visualization of the catalyzed nuclear-spin conversion of molecular hydrogen using energy-selective neutron imaging. J. Phys. Chem. C 2019, 123, 11745–11751. [Google Scholar] [CrossRef] [Green Version]
- ISIS Neutron and Muon Source. Watching Hydrogen Flip Its Spin. Available online: https://www.isis.stfc.ac.uk/Pages/Watching-hydrogen-flip-its-spin.aspx (accessed on 7 March 2021).
- Ossler, F.; Finney, C.E.A.; Warren, J.M.; Bilheux, J.-C.; Zhang, Y.; Mills, R.A.; Santodonato, L.J.; Bilheux, H.Z. Dynamics of hydrogen loss and structural changes in pyrolyzing biomass utilizing neutron imaging. Carbon 2021, 176, 511–529. [Google Scholar] [CrossRef]
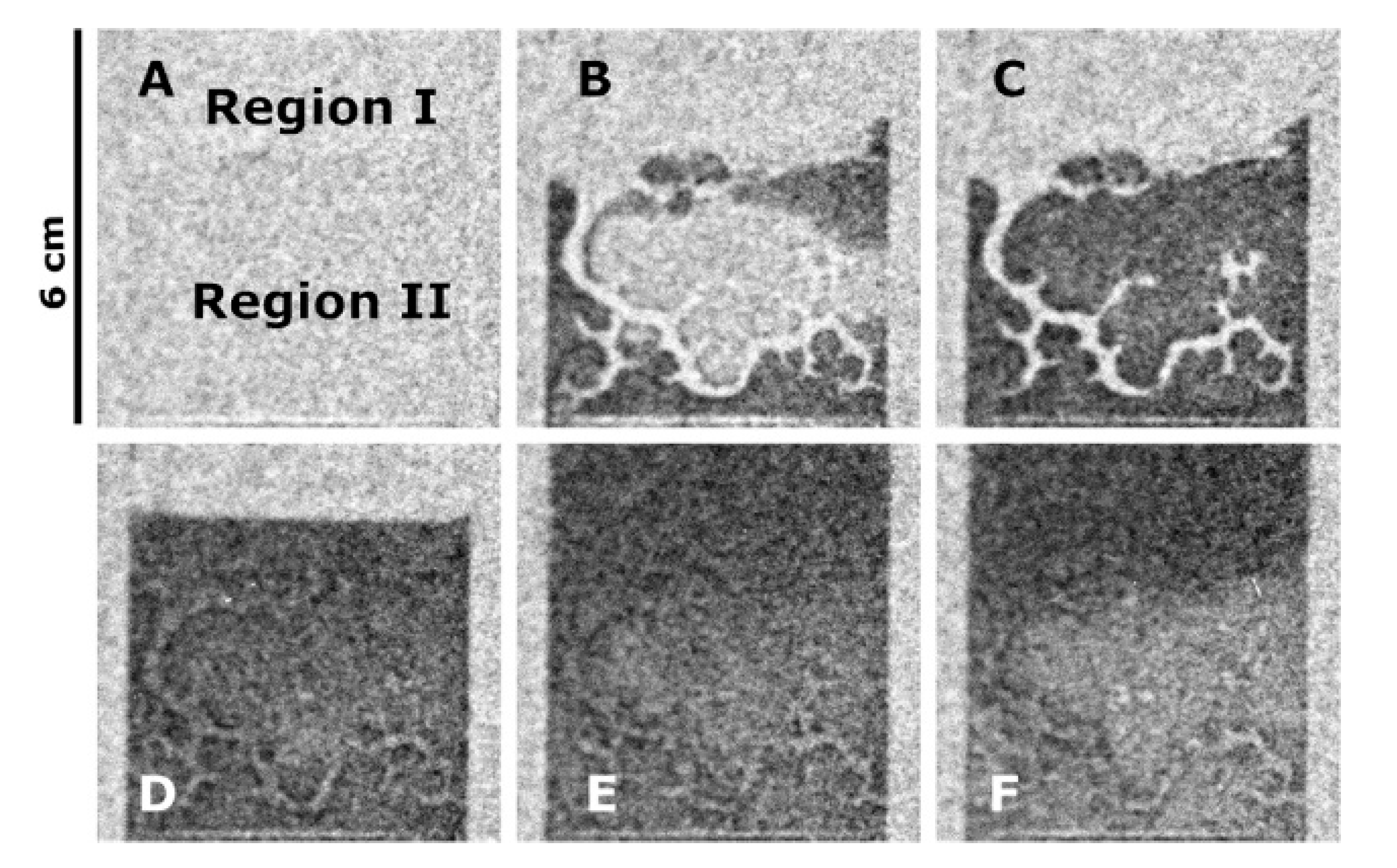
- Toops, T.J.; Bilheux, H.Z.; Voisin, S.; Gregor, J.; Walker, L.; Strzelec, A.; Finney, C.E.A.; Pihl, J.A. Neutron tomography of particulate filters: A non-destructive investigation tool for applied and industrial research. Nucl. Inst. Meth. Phys. Res. A 2013, 729, 581–588. [Google Scholar] [CrossRef]
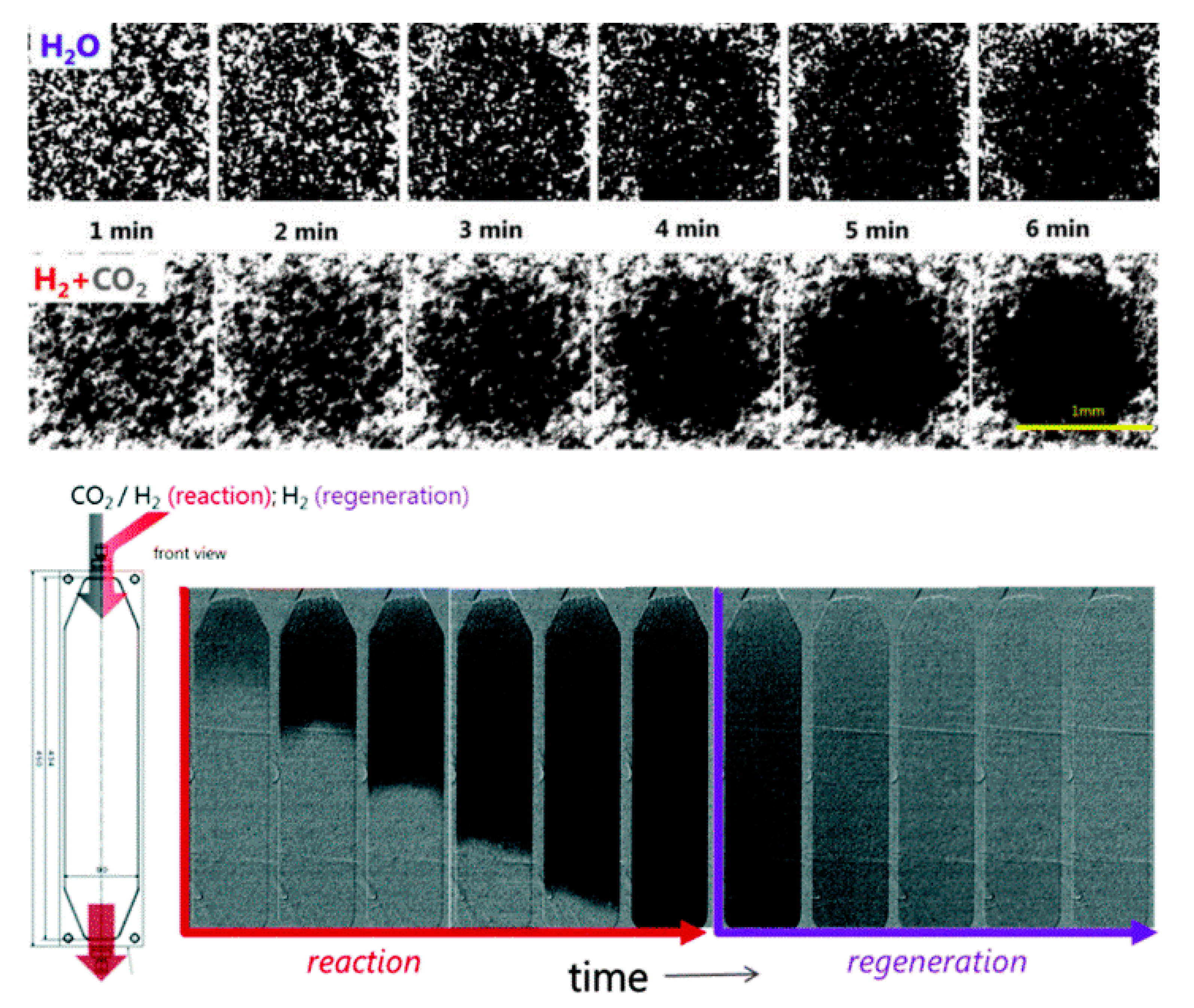
- Borgschulte, A.; Delmelle, R.; Duarte, R.B.; Heel, A.; Boillat, P.; Lehmann, E. Water distribution in a sorption enhanced methanation reactor by time resolved neutron imaging. Phys. Chem. Chem. Phys. 2016, 18, 17217–17223. [Google Scholar] [CrossRef]
- Terreni, J.; Trottmann, M.; Delmelle, R.; Heel, A.; Trtik, P.; Lehmann, E.H.; Borgschulte, A. Observing chemical reactions by time-resolved high-resolution neutron imaging. J. Phys. Chem. C 2018, 122, 23574–23581. [Google Scholar] [CrossRef]
- Terreni, J.; Billeter, E.; Sambalova, O.; Liu, X.; Trottmann, M.; Sterzi, A.; Geerlings, H.; Trtik, P.; Kaestner, A.; Borgschulte, A. Hydrogen in methanol catalysts by neutron imaging. Phys. Chem. Chem. Phys. 2020, 22, 22979–22988. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, E.H. Neutron imaging facilities in a global context. J. Imaging 2017, 3, 52. [Google Scholar] [CrossRef] [Green Version]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 2017, 117, 1445–1514. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef] [PubMed]
- Tseng, I.H.; Chang, W.C.; Wu, J.C.S. Photoreduction of CO2 using sol-gel derived titania and titania-supported copper catalysts. Appl. Catal. B 2002, 37, 37–48. [Google Scholar] [CrossRef]
- Heitmann, T.; Hester, G.; Mitra, S.; Calloway, T.; Tyagi, M.; Miskowiec, A.; Diallo, S.; Osti, N.; Mamontov, E. Probing Li ion dynamics in amorphous xLi2SO4·(1-x)LiPO3 by quasielastic neutron scattering. Solid State Ionics 2019, 334, 95–98. [Google Scholar] [CrossRef]
- Chen, Q.; Jalarvo, N.H.; Lai, W. Na ion dynamics in P2-Nax[Ni1/3Ti2/3]O2: A combination of quasi-elastic neutron scattering and first-principles molecular dynamics study. J. Mater. Chem. A 2020, 8, 25290–25297. [Google Scholar] [CrossRef]
- Howells, W.S.; Barnes, A.C.; Hamilton, M. Quasielastic neutron scattering and the dynamics of Mg2+ in the fast ion and liquid phases of Mg3Bi2. Physica B 1999, 266, 97–99. [Google Scholar] [CrossRef]
- Pinna, R.S.; Rudić, S.; Parker, S.F.; Armstrong, J.; Zanetti, M.; Škoro, G.; Waller, S.P.; Zacek, D.; Smith, C.A.; Capstick, M.J.; et al. The neutron guide upgrade of the TOSCA spectrometer. Nucl. Instrum. Methods Phys. Res. Sect. A 2018, 896, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.H.; Argyriou, D.N.; Jackson, A.J.; Houston, J.; Henry, P.F.; Deen, P.P.; Toft-Petersen, R.; Beran, P.; Strobl, M.; Arnold, T.; et al. The instrument suite of the European Spallation Source. Nucl. Instrum. Methods Phys. Res. Sect. A 2020, 957, 163402–doi10. [Google Scholar] [CrossRef]
- The ISIS Neutron and Muon Source. The ISIS-II Roadmap. Available online: https://www.isis.stfc.ac.uk/Pages/ISIS-II%20Roadmap.pdf#search=ISIS%20II (accessed on 7 March 2021).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, S.F.; Lennon, D. Net Zero and Catalysis: How Neutrons Can Help. Physchem 2021, 1, 95-120. https://0-doi-org.brum.beds.ac.uk/10.3390/physchem1010007
Parker SF, Lennon D. Net Zero and Catalysis: How Neutrons Can Help. Physchem. 2021; 1(1):95-120. https://0-doi-org.brum.beds.ac.uk/10.3390/physchem1010007
Chicago/Turabian StyleParker, Stewart F., and David Lennon. 2021. "Net Zero and Catalysis: How Neutrons Can Help" Physchem 1, no. 1: 95-120. https://0-doi-org.brum.beds.ac.uk/10.3390/physchem1010007





