Thermodynamic Analysis of the V-Shaped Area of High Pressure and High Temperature in Cubic Boron Nitride Synthesis with Li3N as a Catalyst
Abstract
: The possibilities of different phase transitions to cBN with Li3N as catalyst at high temperature and high pressure (1600–2200 K, 4.8–6.0 GPa) are analyzed, in the framework of the second law of thermodynamics. The Gibbs free energy (ΔG) of three reactions which may happen in the Li3N-BN system: hBN + Li3N→Li3BN2, hBN→cBN, and Li3BN2→cBN + Li3N, is calculated, with the influence of high temperature and high pressure on volume included. We show that ΔG of hBN + Li3N→Li3BN2 and hBN→cBN are between −35∼−10 KJ·mol−1 and −25∼−19 KJ·mol−1, respectively. However, ΔG of Li3BN2→cBN + Li3N can be positive or negative. The area formed by the positive data is a V-shaped area, which covers the most part of the cBN growing V-shaped area. It confirms that Li3BN2 is stable in the P-T area of cBN synthesis, and cBN is probably transformed directly from hBN. Analysis suggests that Li3BN2 promotes the transition from hBN to cBN.1. Introduction
High static pressure is the most common method to synthesize cubic boron nitride (cBN) with hexagonal boron nitride (hBN) as raw material and with alkaline metals or alkaline-earth metals and their nitrides as catalysts. The cBN synthesized with lithium nitride (Li3N) is widely used because of its regular crystal shape and suitable particle size. A layer of white powder covers the surface of cBN crystal during the growth. Phase structure characterizations show that the layer is composed of hBN, micro cBN and lithium boron nitrides (Li3BN2) [1]. It is very desirable to study their relationships to understand the phase transition mechanism of cBN as they are in direct contact with the cBN single crystals.
Previous studies indicate that no matter what kind of catalyst is adopted, the temperature and pressure forms a V-shaped area [2–4] where cBN can grow stably. Besides there is a short-range ordered or medium-range ordered structure in high temperature and high pressure (HPHT) system [5], namely a solid microstructure existing in the HPHT system. It was proposed that cBN was directly transformed from hBN [6], while [7] believed that cBN was precipitated from solvent. Thermodynamic analysis plays an important role in understanding the synthesis mechanism of diamond [8,9]. Therefore it is possible to investigate the phase transition mechanism of cBN because of the similar structure. However, there are few reports about the thermodynamics of the cBN transition mechanism with catalyst at HPHT due to the absence of thermodynamic data at HPHT and the ambiguity of the mechanism. Bocquillon et al. [3,10] put forward the following reactions in the Li3N-hBN system:
After the reactions, all of the phase are found around the cBN crystals by XRD [1], except for the volatile Li3N. It is difficult to identify the reactions through experiments because the online inspection is impractical. The synthesis of cBN is an isobaric and isothermal process, and we can apply classical thermodynamics to analyze the reactions in the system by querying the related thermodynamic data. Based on the second law of thermodynamics, we calculate the change of Gibbs free energy in (1) and (2) and analyze the thermodynamic transformation of cBN in V-shaped area of the system.
2. Calculation
The calculation is based on the second law of thermodynamics. The change of Gibbs free energy ΔG is:
Under the isobaric condition, ΔG can be obtained:
ΔG between different temperatures and pressures can be expressed as [11]:
Equation (5) takes the influence of temperature and pressure to volume into consideration. and are enthalpy and entropy of crystalline phase in the system, respectively. V0 is the mole volume at normal temperature and pressure. ΔVT and ΔVP are the variation of mole volume with constant temperature and pressure, respectively. According to [12,13], the related thermodynamic quantities can be acquired. Similarly, we get ΔG of Li3BN2 from [14]:
2.1. ΔVP
Based on the state equation of crystal under high pressure by Sung [15], we use the Birch-Murnaghan equation of crystal state to calculate the variation of volume under high pressure:
In Equation (7)B0 is bulk modulus, and B0′ is the first derivative of the pressure with respect to bulk modulus. From Equation (7), the relation between ΔVP and pressure can be obtained. Table 1 shows the physical parameters of materials in reactions in the Li3N-BN HPHT system.
In Table 1, the B0 and B0′ are not found in references. According to [11], they can be deduced from Vegard’s law from the B0 and B0′ of Li3N and cBN:
In Equation (8)B0 and B1 and B2 are the bulk modulus of Li3BN2, Li3N and cBN, respectively. x1, x2 are the percentage of Li3N and cBN, with x2 = 1 − x1. B0′ could be calculated by the same method. The B0 of Li3BN2 is 248.3 and B0′ is 3.945.
2.2. ΔVT
ΔVT can be obtained on the basis of the thermal expansion properties of crystals:.
In Equation (9), β refers to the volume expansion coefficient, and its value of hBN, cBN and Li3N can be obtained from [19,20], but there has been no report on the value β of Li3BN2. It can be calculated as follows:
From [14] Cp of Li3BN2 can be obtained as follows:
3. Results and Discussion
Different P-T areas of cBN growth in the Li3N-hBN system [2–4] have been proposed, which may be related to the purity and degree of order of the hBN and the synthesis process used. The lowest synthesis pressure is 4.8 GPa and the minimum synthesis temperature is 1690 K at 5.0 GPa in Li3N-hBN system [4], which approximates the V-shaped area proposed in [3]. Ko [7] found that the lowest temperature was 1620 K at 5.3 GPa, lower than the lowest temperature (1723 K) reported by Bocquillon [3]. Considering the industrial application and the reliability of the numerical calculation is performed in an expanded region of 1600–2200 K and 4.8–6.0 GPa.
Table 2 shows the change of Gibbs free energy of Li3N + hBN→Li3BN2 in the HPHT area (1600–2200 K, 4.8–6.0 GPa) of cBN synthesis in the Li3N-hBN system. The values of ΔG are between −35 and −10 KJ·mol−1. This illustrates that Li3N can transform into Li3BN2 in this P-T area, in coincidence with the XRD results of the catalyst after synthesis [1].
ΔG varies regularly with the increase of temperature and pressure. It decreases firstly and then rises with the increasing temperature under constant pressure. ΔG decreases monotonically with the increasing pressure when temperature keeps constant. The higher temperature and pressure, the more negative ΔG, it is easier to generate Li3BN2.The possibility of the reaction exists, although the absolute value of ΔG is very small.
Table 3 shows ΔG of transition hBN-cBN in HPHT area (1600–2200 K, 4.8–6.0 GPa) of cBN synthesis. In consideration of the influence of temperature and pressure on phase volume, relatively precise calculation results are obtained. ΔG of the transition is between −25 and −19 KJ·mol−1. ΔG is negative and its absolute value increases with the increasing temperature and pressure, and it is more and more probable for the transition to occur. From Table 3, we find that ΔG changes more sharply with the pressure than with the temperature. Therefore, the transition of hBN to cBN is probable in the P-T area, and pressure is greater impact than temperature.
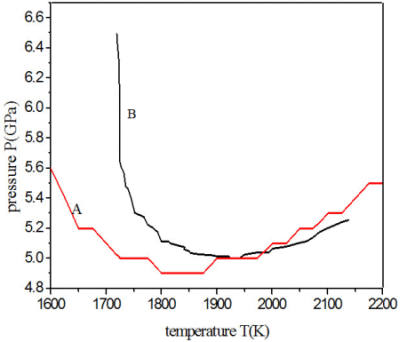
We show the results in Table 4 supposing that Li3BN2 could decompose into cBN. We find that when the pressure is as low as 4.8 GPa, ΔG is negative and ΔG increases from negative to positive with increasing temperature and pressure. ΔG increases from negative to positive with increasing pressure when the temperature is constant. However, ΔG increases first and then decreases with the increasing temperature under constant pressure, and ΔG of reaction Li3BN2→cBN + Li3N can be negative or positive when the pressure is between 4.8 and 6.0 GPa. It can be obtained from Table 4 that the boundary of negative and positive values forms an approximate V-shaped area (the upper area of curve A in Figure 1), in which ΔG of reaction Li3BN2→cBN + Li3N is positive and this reaction does not occur. Therefore Li3BN2 can exist stably, and cBN does not come from the decomposition of Li3BN2 in the V-shaped area. Upper area of Curve B in Figure 1 is the V-shaped area from [8] where cBN can grow stably. Figure 1 indicates that the V-shaped area formed in Table 4 covers the most part of the V-shaped area of cBN growth from the references. In other words, the reaction Li3BN2→cBN + Li3N cannot occur in the V-shaped area where the macroaggregated and high-quality cBN crystals can be obtained. That is to say, cBN cannot be produced by the treatment of only Li3BN2 in the V-shaped area obtained from Table 4. However, it can be seen from Table 3 that in the same area, ΔG of the hBN-cBN transition is negative, and it becomes more and more negative with increasing temperature and pressure. We speculate that in the V-shaped area, cBN is probably transformed directly from hBN.
It is known from [2] that cBN does not form when Li3BN2 melts or decomposes, and only when excess hBN is added can cBN be found. Li3BN2 exists stably in the HPHT area of cBN growth, and Li3BN2 appears to be in equilibrium with cBN in the Li3N-hBN system. We infer that the catalyst promotes the transition of hBN to cBN. The melting point of hBN is over 3000 K at ambient pressure. hBN has a stable structure with a sp2 hybridization state in the hexagonal ring layer and Van der Waals’ force between layers. The hybridized orbital of B atom is sp2 + 2p0, and N atom is 2s2 + 2p2 in hBN. However, the hybridized orbital of B and N atom is sp3 in cBN, and the chemical bond between B and N atom is a stable σ-bond, so the most important driving force for the transition from hBN to cBN is to transfer an electron from N atom to B atom. Part of Li+ in the short-range ordered structure of Li3BN2 absorbs electrons from N atom at the high active state. Because of its instability, Li cannot exist stably in the high temperature and high pressure system. B atoms with empty orbitals absorb the electrons of Li, and then complete the electronic transfer from N to B atoms [21], so both the electrical structures of the B and N atoms are sp2 + 2p1. Electrons in the s orbital of B and N atoms at the high temperature are excited into the empty p orbitals. The B and N atom can form a sp3 hybridization state. B and N atoms in this sp3 hybridization state form σ covalent bonds in a head-to-head mode with energy fluctuations, and generate the microstructure of cBN at high pressure and high temperature.
Li3BN2 is short-range ordered at molten-like state under HTHP, and it can be dissolved into well-ordered hBN. BN23− ion invades hBN and affects the van der Waals forces between the layers, causing inter-layer slippage or breakage. hBN can be disintegrated into two dimensional B-N dusts with low polymerization degree. At this time, the long-range ordered structure disappears, rendering a short-range ordered structure. Without catalyst, higher temperatures than 3000 K and higher pressures than 13 GPa are needed to obtain cBN crystals [4]. With the catalyst, a short-range or medium-range ordered molten-like structure [5] forms to greatly reduce the temperature and pressure of cBN synthesis. From the point of crystal nucleation, the B-N dusts produced by hBN degradation can transform into cBN microstructures by heterogeneous nucleation with energy fluctuation [22]. Li3BN2 could be the matrix of cBN nucleation, because of the similar crystal structure and larger lattice constant [23]. The process only forms the microstructure of cBN crystals. In order to obtain cBN crystals, it is essential for the tiny crystal nucleus to grow gradually [24].
4. Conclusions
(1) In the HPHT area (1600∼2200 K, 4.8∼6.0 GPa) of cBN growth, the reaction hBN + Li3N→Li3BN2 can occur, and the ΔG of the reaction is between −35 and −10 KJ·mol−1. However, in the same P-T area, ΔG of transition hBN→cBN is between −25 and −19 KJ·mol−1, indicating a more probable transition. The ΔG of decomposition Li3BN2→cBN + Li3N is almost positive, thus Li3BN2 can exist stably.
(2) The area of the positive ΔG of Li3BN2 decomposition forms an approximate V-shaped area, which covers the most part of the V-shaped area of cBN crystal growth. Therefore, cBN does not come from the decomposition of Li3BN2 and cBN is probably transformed from hBN. However, analysis shows that Li3BN2 promotes the transfer of an electron from a N atom to a B atom in order to form the micro cBN nuclei.
Acknowledgments
This work is supported by the Natural Science Foundation of China (Grant No. 51272139).
Author Contributions
Bin Xu made substantial contributions to conception and design of this paper. Bin Xu, Mei-Zhe Lv and Hong-Mei Yang performed acquisition, analysis, and interpretation of data. Mei-Zhe Lv and Hong-Mei Yang drafted the paper; Zhen-Xing Wen participated in analysis and interpretation of data, and gave useful suggestions for revising the manuscript; Bin Xu gave final approval of the version to be submitted and any revised version. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, S.; Xu, B.; Guo, X.F.; Wang, H. Fracture morphology and XRD layered characterization of cBN Cake. J. Synth. Cryst 2012, 41, 15–19. (in Chinese). [Google Scholar]
- De Vries, R.C.; Fleischer, J.F. Phase equilibria pertinent to the growth of cubic boron nitride. J. Cryst. Growth 1972, 13, 88–92. [Google Scholar]
- Bocquillon, G.; Loriers, G.; Loriers, J. Synthesis of cubic boron nitride using Mg and pure or M′-doped Li3N, Ca3N2 and Mg3N2 with M′ = Al, B, Si, Ti. J. Mater. Sci 1993, 28, 3547–3556. [Google Scholar]
- Guo, W. Effects of Additive on the Synthesis of High Quality cBN Single Crystal in the Li3N-hBN System (in Chinese). Ph.D. Thesis, Jilin University, Changchun, China. 2011. [Google Scholar]
- Bian, X.F.; Wang, W.M.; Li, H. Metallic Melts Structure (in Chinese); Shanghai Jiao Tong University Press: Shanghai, China, 2003; pp. 66–81. [Google Scholar]
- Xu, X.W.; Zhao, H.M.; Fan, H.L.; Li, Y.P. Synthesis of cBN with hBN. J. Univ. Sci. Technol. Beijing 2001, 23, 337–339. (in Chinese). [Google Scholar]
- Jia, X.R.; Su, Z.P.; Yang, D.P.; Zhang, T. Direct nucleation and growth of cBN by the chemical reaction. Mater. Lett 2008, 62, 1721–1723. [Google Scholar]
- Xu, B.; Li, L.; Tian, B.; Fan, X.H.; Feng, L.M. Thermodynamic analysis of diamond growth with catalyst at HPHT. Chin. J. High Press. Phys 2009, 23, 189–195. (in Chinese). [Google Scholar]
- Lin, M.X.; Li, Y.H. Thick-grain diamond’s growth and ascertainment of its thermodynamic conditions. Chin. J. High Press. Phys 1994, 8, 36–42. (in Chinese). [Google Scholar]
- Ji, X.R. The Effect of hBN Re-crystallized Character on cBN Synthesis and Direct and Growth of cBN (in Chinese). Ph.D. Thesis, Jilin University, Changchun, China. 2008. [Google Scholar]
- Niu, Z.; Xu, B.; Tian, B.; Fan, X.H. Characterization on the diamond/metallic catalyst interface and thermodynamic analysis of the diamond crystal growth. J. Synth. Cryst 2010, 39, 1367–1380. (in Chinese). [Google Scholar]
- Liang, Y.J.; Che, Y.C.; Liu, X.X.; Li, N. J. Thermodynamical Data of Inorganic Substancep (in Chinese); Northeastern University Press: Shenyang, China, 1993; p. 214. [Google Scholar]
- Kuhr, M.; Ke, S.R.; Kulisch, W. Nucleation of cubic boron nitride (c-BN) with ion-induced plasma-enhanced CVD. Int. J. Refract. Met. Hard Mater 1996, 14, 145–157. [Google Scholar]
- Siegel, D.J.; Wolverton, C.; Ozoliņš, V. Reaction energetics and crystal structure of Li4BN3H10 from first principles. Phys. Rev. B 2007, 75, 014101. [Google Scholar]
- Sung, J. Graphite→diamond transition under high pressure: A kinetics approach. J. Mater. Sci 2000, 35, 6041–6054. [Google Scholar]
- Guo, Y.D. Research in Elastic and Thermodynamic Properties for MgO, cBN and other Crystals Under High Pressure (in Chinese). Ph.D. Thesis, Sichuan University, Chengdu, China. 2007. [Google Scholar]
- Solozhenko, V.L.; Turkevich, V.Z. High pressure phase equilibria in the Li3N-BN system: In situ studies. Mater. Lett 1997, 32, 179–184. [Google Scholar]
- Hossain, M.; Islam, A.K.M.A.; Islam, F. Elastic Properties of α-and β-phases of Li3N. J. Sci. Res 2009, 1, 182–191. [Google Scholar]
- Edgar, J.H., Ed.; Properties of Group III Nitrides; EMIS Datareviews Series No. 11; INSPEC, The Institution of Electrical Engineers: London, UK, 1994; pp. 14–15.
- Huq, A.; Richardson, J.W.; Maxey, E.R.; Chandra, D.; Chien, W.-M. Structural studies of Li3N using neutron powder diffraction. J. Alloys Compd 2007, 436, 256–260. [Google Scholar]
- Lu, Z.T. Conversion and inverse transformation of diamond and cubic boron nitride with catalyst. Diam. Abras. Eng 1981, 3, 5–11. (in Chinese). [Google Scholar]
- Wang, C.X. Nucleation thermodynamics of cubic boron nitride upon high-pressure and high-temperature supercritical fluid system in nanoscale. J. Phys. Chem. B 2004, 108, 728–731. [Google Scholar]
- Yamane, H.; Kikkawa, S.; Koizumi, M. High-and low-temperature phases of lithium boron nitride, Li3BN2: Preparation, phase relation, crystal structure, and ionic conductivity. J. Solid State Chem 1987, 71, 1–11. [Google Scholar]
- Guo, X.F.; Xu, B.; Yang, H.M.; Wen, Z.X.; Fan, X.H.; Tian, B. Research on growth interface morphology and phase structures of cBN. J. Synth. Cryst 2012, 41, 1538–1542. (in Chinese). [Google Scholar]

| Material | V0/cm3·mol−1 | B0/GPa | B0′/GPa |
|---|---|---|---|
| cBN | 7.1150 | 398.6 [16] | 3.85 [16] |
| hBN | 10.8820 | 36.7 [17] | 5.6 [17] |
| Li3N | 27.2031 | 98 [18] | 4.04 [18] |
| Li3BN2 | 34.0850 | - | - |
| T/K | ΔG /(KJ·mol−1) | ||||||
|---|---|---|---|---|---|---|---|
| 4.8 GPa | 5.0 GPa | 5.2 GPa | 5.4 GPa | 5.6 GPa | 5.8 GPa | 6.0 GPa | |
| 1600 | −16.4319 | −17.9111 | −19.3804 | −20.8399 | −22.2898 | −23.7301 | −25.1611 |
| 1650 | −17.3670 | −18.9879 | −20.5988 | −22.2000 | −23.7916 | −25.3736 | −26.9463 |
| 1700 | −18.1161 | −19.8872 | −21.6483 | −23.3996 | −25.1413 | −26.8735 | −28.5963 |
| 1750 | −18.6561 | −20.5857 | −22.5054 | −24.4154 | −26.3156 | −28.2064 | −30.0878 |
| 1800 | −18.9630 | −21.0597 | −23.1464 | −25.2233 | −27.2905 | −29.3483 | −31.3967 |
| 1850 | −19.0122 | −21.2842 | −23.5463 | −25.7985 | −28.0411 | −30.2743 | −32.4980 |
| 1900 | −18.7784 | −21.2340 | −23.6797 | −26.1156 | −28.5418 | −30.9586 | −33.3660 |
| 1950 | −18.2357 | −20.8831 | −23.5206 | −26.1482 | −28.7663 | −31.3749 | −33.9740 |
| 2000 | −17.3573 | −20.2046 | −23.0420 | −25.8696 | −28.6875 | −31.4959 | −34.2951 |
| 2050 | −16.1162 | −19.1714 | −22.2166 | −25.2521 | −28.2779 | −31.2942 | −34.3011 |
| 2100 | −14.4845 | −17.7554 | −21.0163 | −24.2674 | −27.5089 | −30.7409 | −33.9634 |
| 2150 | −12.4339 | −15.9281 | −19.4124 | −22.8869 | −26.3518 | −29.8071 | −33.2531 |
| 2200 | −9.9353 | −13.6604 | −17.3756 | −21.0809 | −24.7767 | −28.4629 | −32.1398 |
| T/K | ΔG/(KJ·mol−1) | ||||||
|---|---|---|---|---|---|---|---|
| 4.8 GPa | 5.0 GPa | 5.2 GPa | 5.4 GPa | 5.6 GPa | 5.8 GPa | 6.0 GPa | |
| 1600 | −19.7724 | −20.4187 | −21.0596 | −21.6952 | −22.3255 | −22.9507 | −23.5707 |
| 1650 | −19.7917 | −20.4391 | −21.0810 | −21.7176 | −22.3490 | −22.9752 | −23.5963 |
| 1700 | −19.8295 | −20.4774 | −21.1198 | −21.7569 | −22.3887 | −23.0154 | −23.6370 |
| 1750 | −19.8857 | −20.5334 | −21.1758 | −21.8127 | −22.4445 | −23.0710 | −23.6925 |
| 1800 | −19.9600 | −20.6070 | −21.2485 | −21.8847 | −22.5156 | −23.1414 | −23.7620 |
| 1850 | −20.0523 | −20.6977 | −21.3377 | −21.9723 | −22.6016 | −23.2258 | −23.8450 |
| 1900 | −20.1622 | −20.8052 | −21.4427 | −22.0750 | −22.7019 | −23.3238 | −23.9405 |
| 1950 | −20.2893 | −20.9290 | −21.5633 | −22.1922 | −22.8158 | −23.4343 | −24.0477 |
| 2000 | −20.4333 | −21.0687 | −21.6986 | −22.3232 | −22.9425 | −23.5567 | −24.1657 |
| 2050 | −20.5936 | −21.2235 | −21.8480 | −22.4672 | −23.0811 | −23.6898 | −24.2935 |
| 2100 | −20.7697 | −21.3930 | −22.0109 | −22.6234 | −23.2307 | −23.8328 | −24.4299 |
| 2150 | −20.9609 | −21.5763 | −22.1863 | −22.7909 | −23.3903 | −23.9845 | −24.5736 |
| 2200 | −21.1665 | −21.7726 | −22.3733 | −22.9686 | −23.5587 | −24.1436 | −24.7235 |
| T/K | ΔG/(KJ·mol−1) | ||||||
|---|---|---|---|---|---|---|---|
| 4.8 GPa | 5.0 GPa | 5.2 GPa | 5.4 GPa | 5.6 GPa | 5.8 GPa | 6.0 GPa | |
| 1600 | −3.3405 | −2.4757 | −1.6792 | −0.8553 | −0.0358 | 0.7795 | 1.5904 |
| 1650 | −2.4247 | −1.4193 | −0.4822 | 0.4824 | 1.4426 | 2.3984 | 3.3500 |
| 1700 | −1.7134 | −0.5583 | 0.5285 | 1.6427 | 2.7525 | 3.8580 | 4.9592 |
| 1750 | −1.2296 | 0.0842 | 1.3297 | 2.6026 | 3.8712 | 5.1354 | 6.3953 |
| 1800 | −0.9970 | 0.4846 | 1.8979 | 3.3386 | 4.7750 | 6.2070 | 7.6347 |
| 1850 | −1.0401 | 0.5865 | 2.2086 | 3.8263 | 5.4395 | 7.0484 | 8.6530 |
| 1900 | −1.3837 | 0.4289 | 2.2370 | 4.0406 | 5.8399 | 7.6348 | 9.4254 |
| 1950 | −2.0537 | −0.0459 | 1.9573 | 3.9561 | 5.9505 | 7.9405 | 9.9263 |
| 2000 | −3.0760 | −0.8640 | 1.3434 | 3.5464 | 5.7451 | 7.9393 | 10.1293 |
| 2050 | −4.4774 | −2.0521 | 0.3686 | 2.7849 | 5.1968 | 7.6043 | 10.0076 |
| 2100 | −6.2851 | −3.6376 | −0.9945 | 1.6440 | 4.2783 | 6.9081 | 9.5337 |
| 2150 | −8.5270 | −5.6482 | −2.7739 | 0.0960 | 2.9615 | 5.8226 | 8.6795 |
| 2200 | −11.2312 | −8.1122 | −4.9977 | −1.8877 | 1.2180 | 4.3193 | 7.4164 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, B.; Lv, M.-Z.; Yang, H.-M.; Wen, Z.-X. Thermodynamic Analysis of the V-Shaped Area of High Pressure and High Temperature in Cubic Boron Nitride Synthesis with Li3N as a Catalyst. Entropy 2014, 16, 912-920. https://0-doi-org.brum.beds.ac.uk/10.3390/e16020912
Xu B, Lv M-Z, Yang H-M, Wen Z-X. Thermodynamic Analysis of the V-Shaped Area of High Pressure and High Temperature in Cubic Boron Nitride Synthesis with Li3N as a Catalyst. Entropy. 2014; 16(2):912-920. https://0-doi-org.brum.beds.ac.uk/10.3390/e16020912
Chicago/Turabian StyleXu, Bin, Mei-Zhe Lv, Hong-Mei Yang, and Zhen-Xing Wen. 2014. "Thermodynamic Analysis of the V-Shaped Area of High Pressure and High Temperature in Cubic Boron Nitride Synthesis with Li3N as a Catalyst" Entropy 16, no. 2: 912-920. https://0-doi-org.brum.beds.ac.uk/10.3390/e16020912