Biosynthesized Silica Nanosuspension as Thermal Fluid in Parabolic Solar Panels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silica Nanoparticles Biosynthesis
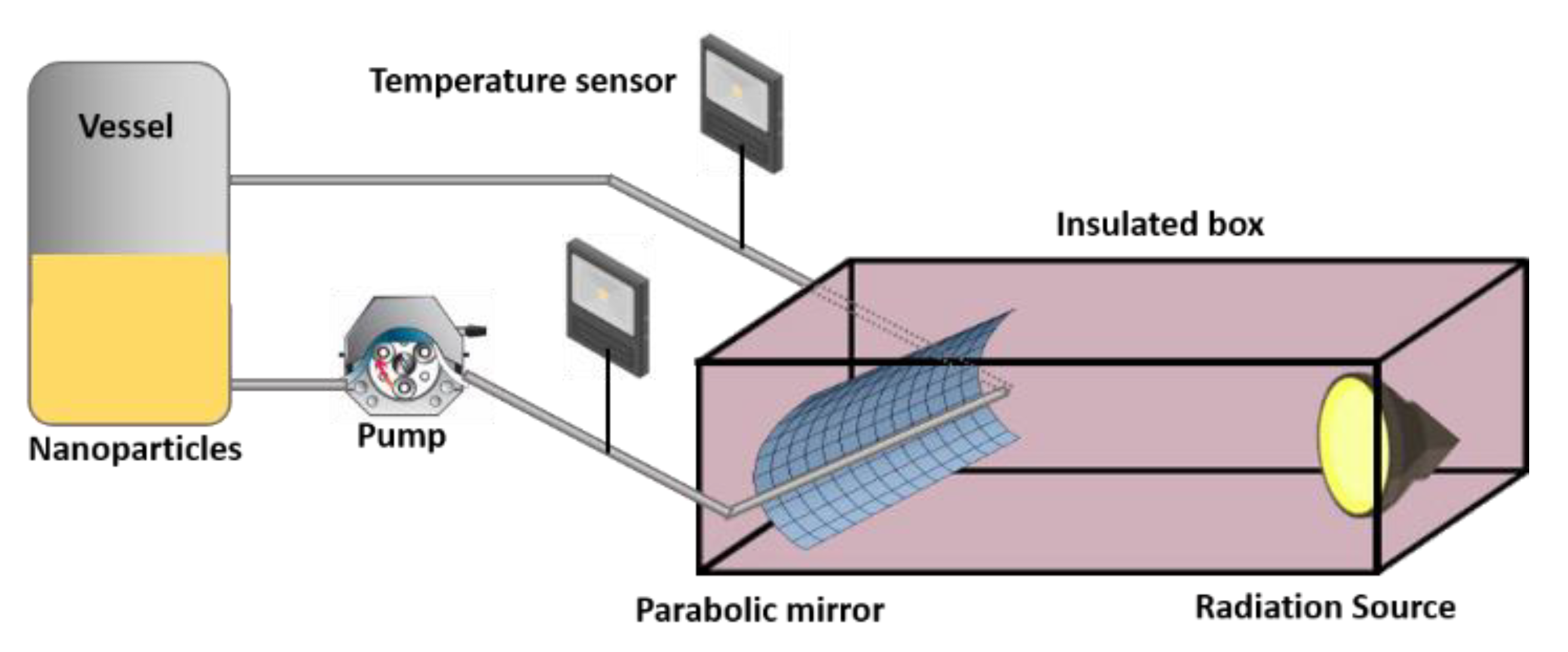
2.2. Solar Panel Bench
3. Results and Discussion
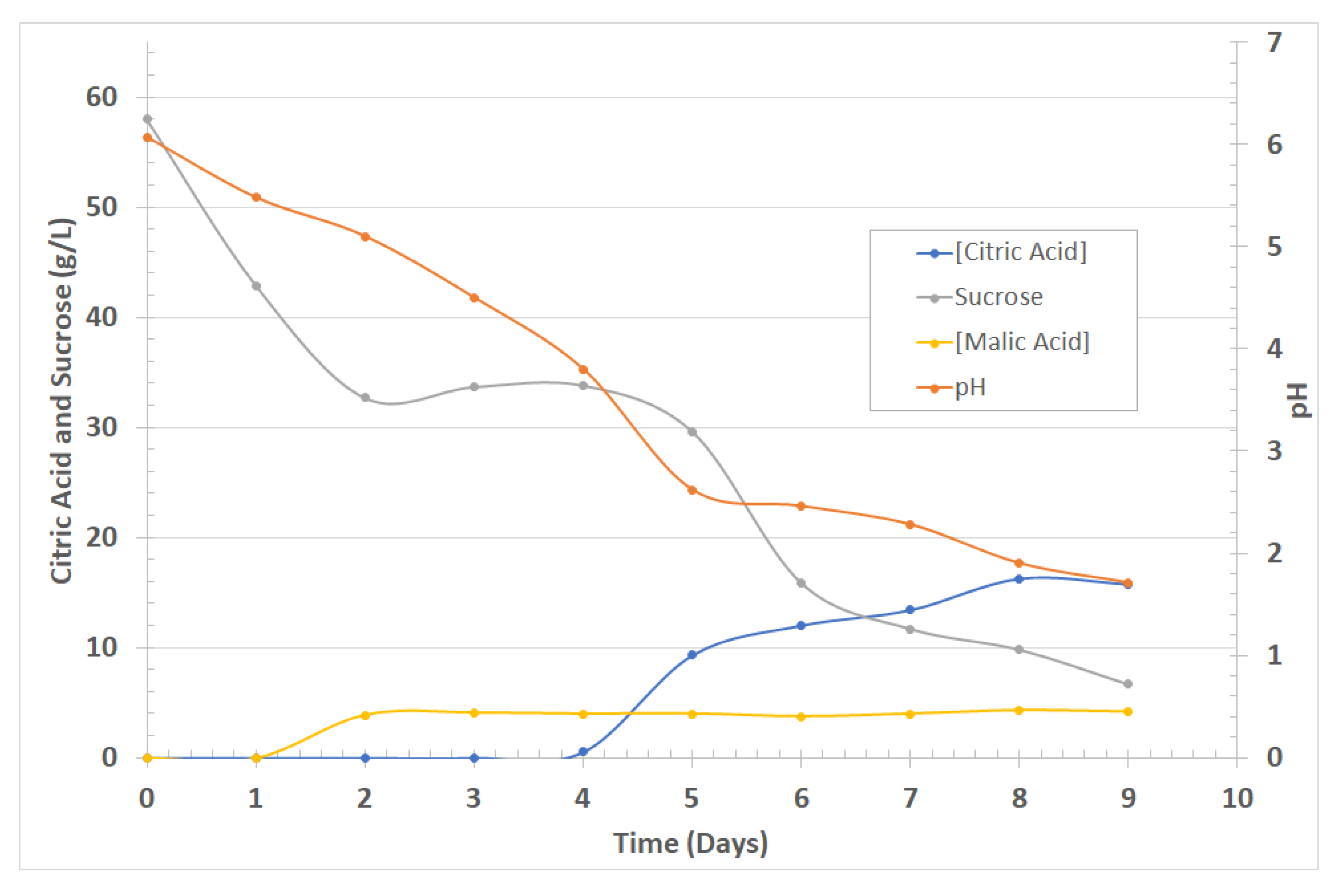
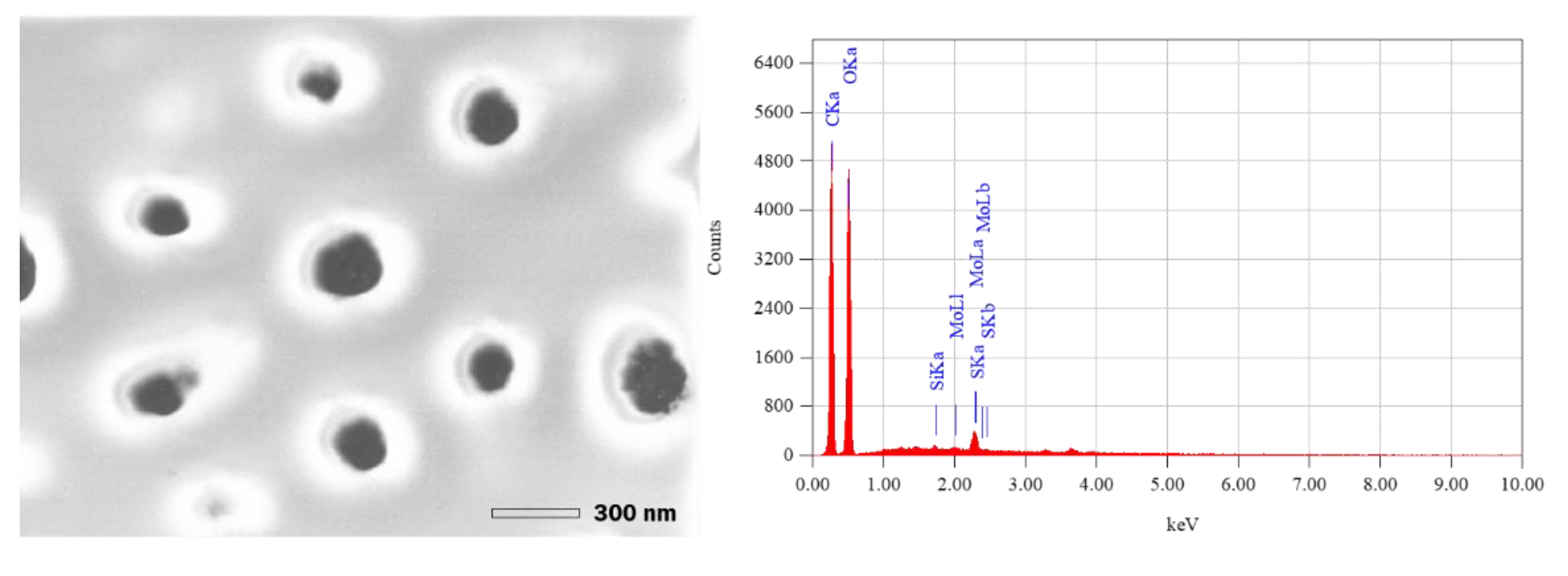
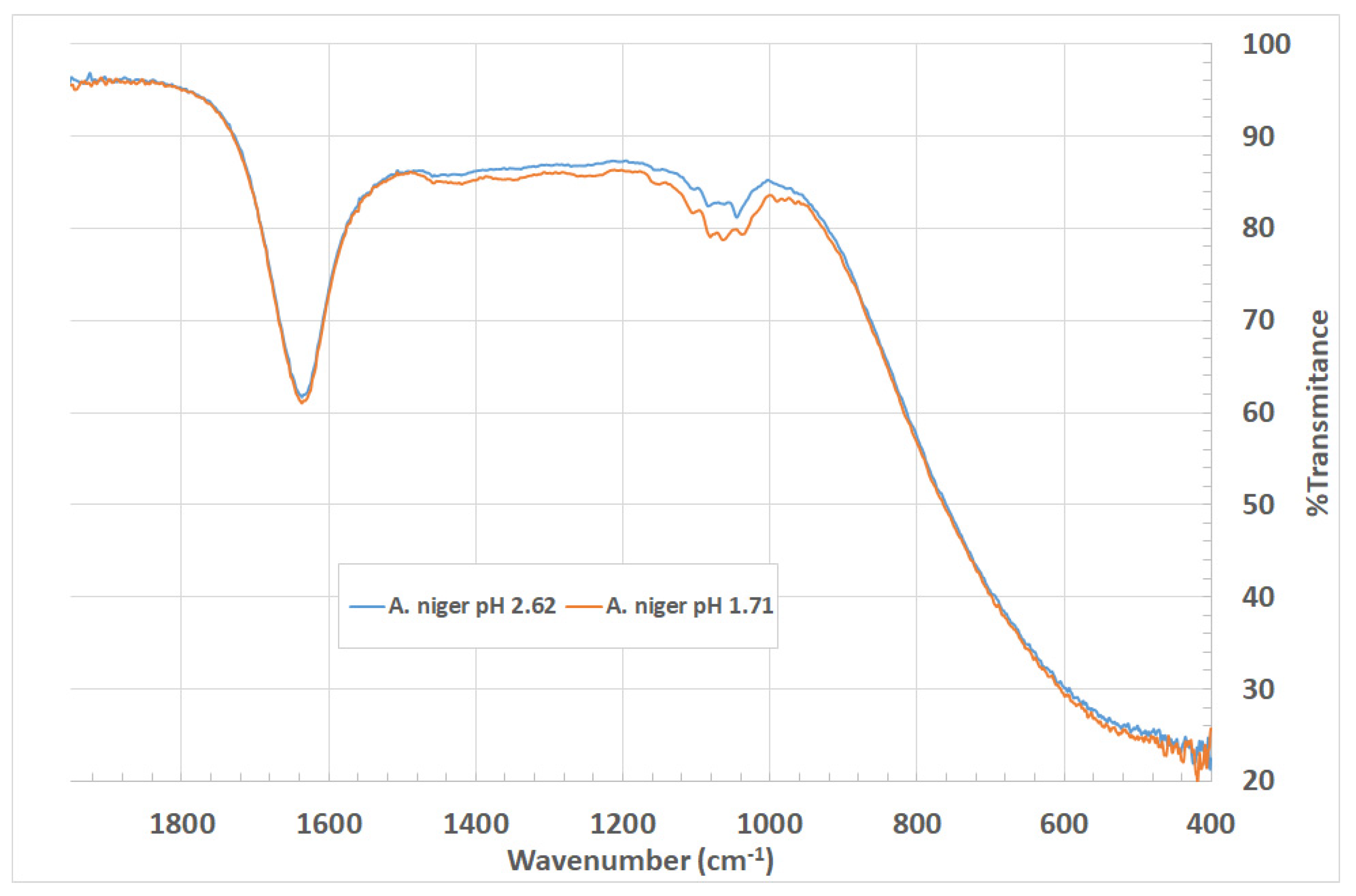
3.1. Silica Nanoparticles Biosynthesis
3.2. Parabolic Solar Panels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, R.; Kaveh, R.K. Renewable Hybridization of Oil and Gas Supply Chains. In Polygeneration with Polystorage for Chemical and Energy Hubs; Khalilpour, K.R., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 331–372. [Google Scholar]
- Neves, S.A.; Marques, A.C.; Fuinhas, J.A. On the drivers of peak electricity demand: What is the role played by battery electric cars? Energy 2018, 159, 905–915. [Google Scholar] [CrossRef]
- Sadeq, J.; Najjar, Y. Modeling and Simulation of Solar Thermal Power System Using Parabolic Trough Collector. Energy Eng. 2017, 143, 04016056. [Google Scholar]
- Broumand, M.; Albert-Green, S.; Yun, S.; Hong, Z.; Thomson, M.J. Spray combustion of fast pyrolysis bio-oils: Applications, challenges, and potential solutions. Prog. Energy Combust. Sci. 2020, 79, 100834. [Google Scholar] [CrossRef]
- Singh, O.; Singh, B.R. Global Trends of Fossil Fuel Reserves and Climate Change in the 21st Century. In Fossil Fuel and the Environment; Khan, S., Ed.; IntechOpen: London, UK, 2016; pp. 167–191. [Google Scholar]
- Abokyi, E.; Appiah-Konadu, P.; Abokyi, F.; Oteng-Abayie, E.F. Industrial growth and emissions of CO2 in Ghana: The role of financial development and fossil fuel consumption. Energy Rep. 2019, 5, 1339–1353. [Google Scholar] [CrossRef]
- International Energy Agency. OECD Green Growth Studies: Energy? OCDE Publishing: París, France, 2011. [Google Scholar]
- Sorknæs, P.; Lund, H.; Skov, I.; Djørup, S.; Skytte, K.; Morthorst, P.; Fausto, F. Smart Energy Markets―Future electricity, gas and heating markets. Renew. Sustain. Energy 2020, 119, 109655. [Google Scholar] [CrossRef]
- Daabo, A.M.; Hammo, K.E.; Mohammed, O.A.; Hassan, A.A.; Lattimore, T. Performance investigation and design optimization of micro scale compressed air axial turbine for domestic solar powered Brayton cycle. Sustain. Energy Technol. Assess. 2020, 37, 100583. [Google Scholar] [CrossRef]
- Rao, Z.; Xu, T.; Liu, C.; Zheng, Z.; Liang, L.; Hong, K. Experimental study on thermal properties and thermal performance of eutectic hydrated salts/expanded perlite form-stable phase change materials for passive solar energy utilization. Sol. Energy Mater. Sol. Cells 2018, 188, 6–17. [Google Scholar] [CrossRef]
- Huang, H.L.; Huang, Z.H.; Chu, Y.C.; Lin, H.P.; Chang, Y.J. Application of metallic nanoparticle-biochars with ionic liquids for thermal transfer fluids. Chemosphere 2020, 250, 126219. [Google Scholar] [CrossRef]
- Li, Y.; Kalbasi, R.; Nguyen, Q.; Afrand, M. Effects of sonication duration and nanoparticles concentration on thermal conductivity of silica-ethylene glycol nanofluid under different temperatures: An experimental study. Powder Technol. 2020, 367, 404–473. [Google Scholar] [CrossRef]
- Pourrajab, R.; Noghrehabadi, A.; Hajidavalloo, E.; Behbahani, M. Investigation of thermal conductivity of a new hybrid nanofluids based on mesoporous silica modified with copper nanoparticles: Synthesis, characterization and experimental study. J. Mol. Liq. 2020, 300, 112337. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Gun-Do, K.; Prakash Patila, M. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 487–495. [Google Scholar]
- Haragobinda, S.; Ranjan Kumar, M.; Pankaj Kumar, P. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar]
- Mohanan Pillai, A.; Sankar Sivasankarapillai, V.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Anuf, R. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Ovais, M.; Talha Khalil, A.; Ayaz, M.; Ahmad, I.; Kumar Nethi, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [Green Version]
- Papagianni, M. Advances in citric acid fermentation by Aspergillus niger: Biochemical aspects, membrane transport and modeling. Biotechnol. Adv. 2007, 25, 244–263. [Google Scholar] [CrossRef] [PubMed]
- Hotza, D.; Ramírez-Carmona, M.; Pineda-Vásquez, T.; Casas-Botero, A.; Torres-Taborda, M.; Soares, C.L. Biogeneration of Silica Nanoparticles from Rice Husk Ash Using Fusarium oxysporum in Two Different Growth Media. Ind. Eng. Chem. Res. 2014, 53, 6959–6965. [Google Scholar]
- Bait, O. Direct and indirect solar–powered desalination processes loaded with nanoparticles: A review. Sustain. Energy Technol. Assess. 2020, 37, 100597. [Google Scholar] [CrossRef]
- Muddanna, M.H.; Baral, S.S. A comparative study of the extraction of metals from the spent fluid catalytic cracking catalyst using chemical leaching and bioleaching by Aspergillus niger. J. Environ. Chem. Eng. 2019, 7, 103335. [Google Scholar] [CrossRef]
- Castro, I.D.M.; Fietto, J.L.R.; Vieira, R.X.; Trópia, M.J.M.; Campos, L.M.M.D.; Paniago, E.B.; Brandão, R.L. Bioleaching of zinc and nickel from silicates using Aspergillus niger cultures. Hydrometallurgy 2000, 57, 39–49. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Luo, Q.; Wang, Q.; Wu, T. Biosorption behavior of heavy metals in bioleaching process of MSWI fly ash by Aspergillus niger. Biochem. Eng. J. 2009, 46, 294–299. [Google Scholar] [CrossRef]
- Bahaloo-Hore, N.; Mousavi, S.M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef] [Green Version]
- Chaerun, S.K.; Sulistyo, R.S.; Minwal, W.P.; Mubarok, M.Z. Indirect bioleaching of low-grade nickel limonite and saprolite ores using fungal metabolic organic acids generated by Aspergillus niger. Hydrometallurgy 2017, 174, 29–37. [Google Scholar] [CrossRef]
- Esmaeili, M.; Rastegar, S.; Beigzadeh, R.; Gu, T. Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2. Chemosphere 2020, 254, 126670. [Google Scholar] [CrossRef]
- Bailey, P.S.J.; Bailey, C.A. Química Orgánica: Conceptos y Aplicaciones; Prentice Hall Hispanoamericana: Ciudad de México, México, 1998. [Google Scholar]
- McMurry, J. Química Orgánica; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Ascensio, J.S. Equilibrios Químicos; Visión Libros: Madrid, Spain, 2013. [Google Scholar]
- Zafar, Z.I.; Ashraf, M. Selective leaching kinetics of calcareous phosphate rock in lactic acid. Chem. Eng. J. 2007, 131, 41–48. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ahn, J.G.; Rhee, Y.H. Bioleaching: A microbial process of metal recovery; A review. Met. Mater. Int. 2005, 11, 249–256. [Google Scholar] [CrossRef]
- Cyrille, A.D.; Edith, K.K.; Marius, N.P.; Bilé, A.; Bernadette, E.; Henri, A.K. Experimental and Theoretical Studies of Oxalic Acid Dissociation in Water-Ethanol Solvents. Int. J. Sci. Res. 2015, 4, 280–286. [Google Scholar]
- Gadd, G.M. Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes. Adv. Microb. Physiol. 1999, 41, 47–92. [Google Scholar]
- Burgstaller, W.; Müller, B.; Schinner, F. The in vivo effect of glucose and extracellular pH on the plasma membrane H+-ATPase of Penicillium simplicissimum. FEMS Microbiol. Lett. 1997, 147, 109–114. [Google Scholar] [CrossRef]
- Li, E.; Wang, C.; Guo, Z.; Haoran, Z. Effects of silane coupling agents on the electrical properties of silica/epoxy nanocomposites. Mater. Sci. 2016, 2, 1036–1039. [Google Scholar]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, P.; Rajeswari, V.D. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corzo-Deluquez, E.; Pineda-Muñoz, L.; Ruíz-Chamorro, A.; Ocampo-López, C.; Ramírez-Carmona, M.; Rendón-Castrillón, L. Biosynthesized Silica Nanosuspension as Thermal Fluid in Parabolic Solar Panels. Entropy 2021, 23, 142. https://0-doi-org.brum.beds.ac.uk/10.3390/e23020142
Corzo-Deluquez E, Pineda-Muñoz L, Ruíz-Chamorro A, Ocampo-López C, Ramírez-Carmona M, Rendón-Castrillón L. Biosynthesized Silica Nanosuspension as Thermal Fluid in Parabolic Solar Panels. Entropy. 2021; 23(2):142. https://0-doi-org.brum.beds.ac.uk/10.3390/e23020142
Chicago/Turabian StyleCorzo-Deluquez, Enrique, Lina Pineda-Muñoz, Adiela Ruíz-Chamorro, Carlos Ocampo-López, Margarita Ramírez-Carmona, and Leidy Rendón-Castrillón. 2021. "Biosynthesized Silica Nanosuspension as Thermal Fluid in Parabolic Solar Panels" Entropy 23, no. 2: 142. https://0-doi-org.brum.beds.ac.uk/10.3390/e23020142









