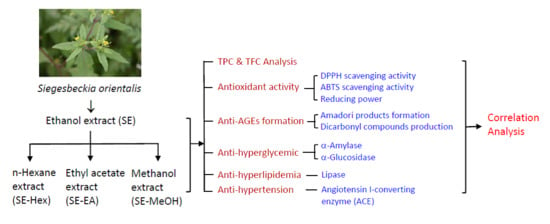
Inhibitory Effects of Siegesbeckia orientalis Extracts on Advanced Glycation End Product Formation and Key Enzymes Related to Metabolic Syndrome
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Polyphenol and Flavonioid Content of S. orientalis Extracts
2.2. Antioxidant Effects
2.3. Inhibitory Effects on AGEs Formation
2.4. Inhibitory Effects on Carbohydrate-Hydrolyzing Enzymes
2.5. Inhibitory Effect on Lipase
2.6. Inhibitory Effect on ACE
2.7. Chemical Composition of S. orientalis Extracts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of S. orientalis Extracts
3.3. TPC and TFC Analysis [51]
3.4. Antioxidant Activity Assay
3.4.1. Scavenging Activity on DPPH Radicals [51]
3.4.2. Scavenging Activity on ABTS Radicals [70]
3.4.3. Reducing Power Assay [71]
3.5. Inhibitory Activity on AGEs Formation [47,52]
3.5.1. Nitroblue Tetrazolium (NBT) Reductive Assay
3.5.2. Girard-T Assay
3.6. Antiglycemic Assays [44]
3.6.1. Assay of α-Glucosidase Activity
3.6.2. Assay of α-Amylase Activity
3.7. Assay of Lipase Activity [72]
3.8. Assay of ACE Activity [73]
3.9. Analysis of Chemical Compositions by HPLC
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| ACE | Angiotensin converting enzyme |
| AGEs | Advanced glycated end products |
| BHT | 2,6-Bis(1,1-dimethylethyl)-4-methylphenol |
| BSA | Bovine serum albumin |
| DNS | 3,5-Dinitrosalicylic acid |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| DTT | d,l-Dithiothreitol |
| HHL | Hippuryl-His-Leu acetate salt |
| NBT | Nitroblue tetrazolium |
| PNPG | 4-Nitrophenyl-α-d-glucopyranoside |
| ROS | Reactive oxygen species |
| RT | Retention time |
| SE | Ethanol extract of S. orientalis |
| SE-EA | Ethyl acetate extract of S. orientalis |
| SE-Hex | n-Hexane extract of S. orientalis |
| SE-MeOH | methanol extract of S. orientalis |
| TCA | Trichloroacetic acid |
| TFC | Total flavonoids content |
| TPC | Total polyphenols content |
References
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Sathyaprakash, R.; Henry, R.R. Preventing diabetes by treating aspects of the metabolic syndrome. Curr. Diabetes Rep. 2002, 2, 416–422. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Apostolidis, E.; Genovese, M.I.; Lajolo, F.M.; Shetty, K. Evaluation of indigenous grains from the Peruvian Andean region for antidiabetes and antihypertension potential using in vitro methods. J. Med. Food 2009, 12, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and α-glucosidase inhibitory activity of colored grains in China. J. Agric. Food Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Wendt, T.; Bucciarelli, L.; Qu, W.; Lu, Y.; Yan, S.F.; Stern, D.M.; Schmidt, A.M. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: Insights into the pathogenesis of macrovascular complications in diabetes. Curr. Atheroscler. Rep. 2002, 4, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Takeuchi, M.; Inagaki, Y.; Nakamura, K.; Imaizumi, T. Role of advanced glycation end products (AGEs) and their receptor (RAGE) in the pathogenesis of diabetic microangiopathy. Int. J. Clin. Pharm. Res. 2003, 23, 129–134. [Google Scholar]
- Yamagishi, S.; Imaizumi, T. Diabetic vascular complications: Pathophysiology, biochemical basis and potential therapeutic strategy. Curr. Pharm. Des. 2005, 11, 2279–2299. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Yamagishi, S.; Matsui, T.; Nakamura, K.; Imaizumi, T. Role of advanced glycation end products (AGEs) in thrombogenic abnormalities in diabetes. Curr. Neurovasc. Res. 2006, 3, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F.J. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Cassel, C.K.; Leipzig, R.M.; Cohen, H.J.; Larson, E.B.; Meier, D.E. Geriatric Medicine, 3rd ed.; Springer: New York, NY, USA, 1997; pp. 7–25. [Google Scholar]
- Katayama, S.; Haga, Y.; Saeki, H. Loss of filament-forming ability of myosin by non-enzymatic glycosylation and its molecular mechanism. FEBS Lett. 2004, 575, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.I.A.; Souza, E.M.; Pedrosa, F.O.; Réa, R.R.; Alves, A.S.C.; Picheth, G.; Rego, F.G.M. RAGE receptor and its soluble isoforms in diabetes mellitus complications. J. Bras. Patol. Med. Lab. 2013, 49, 97–108. [Google Scholar] [CrossRef]
- Tsuji-Naito, K.; Saeki, H.; Hamano, M. Inhibitory effects of Chrysanthemum species extracts on formation of advanced glycation end-products. Food Chem. 2009, 116, 854–859. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mustar, S.; Khalid, N.M.; Rashed, A.; Noh, M.F.M.; Wilcox, M.; Chater, P.; Brownlee, I.; Pearson, J.P. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol. 2013, 25, 1405–1412. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.; Lara, J.; O’Hill, J. ABC of obesity: Strategies for preventing obesity. Br. Med. J. 2006, 333, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.S.; Nieuwdorp, M.; Jukema, J.W.; Kastelein, J.J. Cardiovascular metabolic syndrome—An interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes. Metab. 2007, 9, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, Y.E.; Linas, S. The role of obesity in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2009, 5, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G.; Burn, P. Lipid metabolic enzymes: Emerging drug targets for the treatment of obesity. Nat. Rev. Drug Discov. 2004, 3, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O. Shaddock peels (Citrus maxima) phenolic extracts inhibit α-amylase, α-glucosidase and angiotensin I-converting enzyme activities: A nutraceutical approach to diabetes management. Diabetes Metab. Syndr. 2011, 5, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Sowers, J.R. Diabetes mellitus and hypertension. Hypertension 1992, 19, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard-Larsen, P.; Bundgaard, H. A Textbook of Drug Design and Development; Harwood Academic Publishers: Chur, Switzerland, 1991; pp. 302–307. [Google Scholar]
- Erdos, E.G.; Skidgel, R.A. The angiotensin I-converting enzyme. Lab. Investig. 1987, 56, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Wang, H. Immunosuppressive activity of the ethanol extract of Siegesbeckia orientalis on the immune responses to ovalbumin in mice. Chem. Biodivers. 2006, 3, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Luo, Q.; Ruan, J.L.; Chen, Y.A.; Chen, M.X. Effect of Siegesbeckia orientalis L. on cervical cancer HeLa cell in vitro. Her. Med. 2009, 28, 45–46. [Google Scholar]
- Chang, C.C.; Hsu, H.F.; Huang, K.H.; Wu, J.M.; Kuo, S.M.; Ling, X.H.; Houng, J.Y. Anti-proliferative effects of Siegesbeckia orientalis ethanol extract on human endometrial RL-95 cancer cells. Molecules 2014, 19, 19980–19994. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Ling, X.H.; Hsu, H.F.; Wu, J.M.; Wang, C.P.; Yang, J.F.; Fang, L.W.; Houng, J.Y. Siegesbeckia orientalis extract inhibits TGFβ1-induced migration and invasion of endometrial cancer cells. Molecules 2016, 21, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Weng, L.W.; Chang, C.C.; Hsu, H.F.; Wang, C.P.; Wang, S.W.; Houng, J.Y. Anti-inflammatory effects of Siegesbeckia orientalis ethanol extract in in vitro and in vivo models. BioMed Res. Int. 2014, 329712. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Thuong, P.T.; Hwang, I.H.; Hoang, T.K.; Nguyen, M.K.; Nguyen, H.A.; Na, M. Anti-hyperuricemic, anti-inflammatory and analgesic effects of Siegesbeckia orientalis L. Resulting from the fraction with high phenolic content. BMC Complement. Altern. Med. 2017, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Satya, S.; Khandelwal, R.K.; Naik, S.N. Commonly consumed Indian plant food materials in the management of diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2010, 4, 21–40. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sheliya, M.A.; Rayhana, B.; Ali, A.; Pillai, K.K.; Aeri, V.; Sharma, M.; Mir, S.R. Inhibition of α-glucosidase by new prenylated flavonoids from Euphorbia hirta L. herb. J. Ethnopharmacol. 2015, 176, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Suresh, B.P.; Srinivasan, K. Amelioration of renal lesions associated with diabetes by dietary curcumin in streptootocin diabetic rats. Mol. Cell. Biochem. 1998, 181, 87–96. [Google Scholar]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Current understanding of dietary polyphenols and their role in health and disease. Curr. Nutr. Food Sci. 2009, 5, 249–263. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Recent advances in health promoting effect of dietary polyphenols. Curr. Nutr. Food Sci. 2012, 8, 254–264. [Google Scholar] [CrossRef]
- Anhea, F.F.; Desjardinsb, Y.; Pilona, G.; Dudonneb, S.; Genovesec, M.I.; Lajoloc, F.M. Polyphenols and type 2 diabetes: A prospective review. PharmaNutrition 2013, 1, 105–114. [Google Scholar] [CrossRef]
- Palanisamy, U.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid Isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]
- Wu, J.W.; Hsieh, C.L.; Wang, H.Y.; Chen, H.Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Manaharan, T.; Teng, L.L.; Appleton, D.; Ming, C.H.; Masilamani, T.; Palanisamy, U.D. Antioxidant and antiglycemic potential of Peltophorum pterocarpum plant parts. Food Chem. 2011, 129, 1355–1361. [Google Scholar] [CrossRef]
- Manaharan, T.; Appleton, D.; Cheng, H.M.; Palanisamy, U.D. Flavonoids isolated from Syzygium aqueum leaf extract as potential antihyperglycaemic agents. Food Chem. 2012, 132, 1802–1807. [Google Scholar] [CrossRef]
- Dong, H.Q.; Li, M.; Zhu, F.; Liu, F.L.; Huang, J.B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Lushchak, V.I. Freeradicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.D.; Hsu, H.F.; Chen, Z.H.; Wang, Y.T.; Huang, S.H.; Chen, H.J.; Wang, C.P.; Wang, S.W.; Chang, C.C.; Houng, J.Y. Antioxidant, anti-inflammatory, and anti-proliferative activities of extracts from different parts of farmed and wild Glossogyne tenuifolia. Ind. Crop. Prod. 2014, 57, 98–105. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wang, X.; Dong, L.L. Antioxidation and antiglycation of polysaccharides from Misgurnus anguillicaudatus. Food Chem. 2011, 124, 183–187. [Google Scholar] [CrossRef]
- Kwon, G.J.; Choi, D.S.; Wang, M.H. Biological activities of hot water extracts from Euonymus alatus leaf. Korean J. Sci. Technol. 2007, 39, 569–574. [Google Scholar]
- Oboh, G.; Akinyemi, A.J.; Ademiluyi, A.O. Inhibition of α-amylase and α-glucosidase activities by ethanolic extract of Telfairia occidentalis (fluted pumpkin) leaf. Asian Pac. J. Trop. Biomed. 2012, 2, 733–738. [Google Scholar] [CrossRef]
- Jung, H.A.; Yung, Y.J.; Na, Y.Y.; Jeong, D.M.; Bae, H.J.; Kim, D.W.; Na, D.H.; Choi, J.S. Inhibitory effects of Nelumbo nucifera leaves on rat lens aldosereductase, advanced glycation endproducts formation, and oxidative stress. Food Chem. Toxicol. 2008, 46, 3818–3826. [Google Scholar] [CrossRef] [PubMed]
- Cheplick, S.; Kwon, Y.I.; Bhowmik, P.; Shetty, K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresour. Technol. 2010, 101, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.D.; Padhi, E.; Hawke, A.; Sivaramalingam, T.; Tsao, R. The glycemic index of pigmented potatoes is related to their polyphenol content. Food Funct. 2014, 5, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Worsztynowicza, P.; Napierała, M.; Białasa, W.; Grajeka, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Franck, U.; Wagner, H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O. Phenolic extracts from grape fruit peels (Citrus paradisi) inhibit key enzymes linked with type 2 diabetes and hypertension. J. Food Biochem. 2011, 35, 1703–1709. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Phenolic compounds in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia and hypertension. Food Chem. 2012, 133, 156–162. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Syama, H.P.; Arya, A.D.; Dhanya, R.; Nisha, P.; Sundaresan, A.; Jacob, E.; Jayamurthy, P. Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. J. Food Sci. Technol. 2017, 54, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Isaac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014, 10, 208–216. [Google Scholar] [PubMed]
- Hsu, H.F.; Chang, S.F.; Chen, Z.H.; Yuan, S.S.F.; Tsai, Y.D.; Wang, C.P.; Wang, S.W.; Fang, L.W.; Houng, J.Y. Cytotoxic effect of Anisomeles indica extract on human pharynx squamous cancer cells. J. Med. Plants Res. 2012, 6, 5002–5012. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.H. Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- McDougall, G.J.; Nimish, N.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
Sample Availability: No samples or compounds are available from the authors. |









| Parameter | Antioxidation | Anti-AGEs Formation | Antihyperglycemic | Antihyper-Lipidemia | Antihyper-Tension | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1/IC50, DPPH | 1/IC50, ABTS | Reducing Power | NBT Reduction | Girard-T Assay | 1/IC50, amy | 1/IC50, glu | 1/IC50, PL | 1/IC50, ACE | |
| TPC | 0.993 | 0.925 | 0.975 | −0.346 | 0.804 | - | 0.945 | 0.945 | 0.983 |
| TFC | 0.907 | 0.829 | 0.794 | 0.042 | 0.585 | - | 0.650 | 0.813 | 0.926 |
| 1/IC50, DPPH | 1 | 0.953 | 0.944 | −0.337 | 0.735 | - | 0.919 | 0.910 | 0.978 |
| 1/IC50, ABTS | 1 | 0.828 | −0.522 | 0.530 | - | 0.949 | 0.750 | 0.870 | |
| Reducing power | 1 | −0.311 | 0.914 | - | 0.977 | 0.985 | 0.965 | ||
| NBT reduction | 1 | −0.121 | - | −0.797 | −0.146 | −0.170 | |||
| Girard-T assay | 1 | - | 1.000 | 0.943 | 0.823 | ||||
| 1/IC50, amy | 1 | - | - | - | |||||
| 1/IC50, glu | 1 | 0.847 | 0.686 | ||||||
| 1/IC50, PL | 1 | 0.965 | |||||||
| 1/IC50, ACE | 1 | ||||||||
| Sample | IC50 (μg/mL) | Ki * (μg/mL) | Inhibition Type |
|---|---|---|---|
| SE-EA | 626.6 | 794.5 | Competitive |
| Captopril | 2.69 × 10−3 | 1.0 × 10−3 | Competitive |
| Peak No. | Compound | Concentration (mg/g Extract) | |||
|---|---|---|---|---|---|
| SE-MeOH | SE-Hex | SE-EA | SE | ||
| 1 | Chlorogenic acid | 0.95 ± 0.05 | - b | 0.72 ± 0.04 | 0.98 ± 0.04 |
| 2 | Syringic acid | TA a | TA a | 2.21 ± 0.11 | 0.26 ± 0.02 |
| 3 | p-Coumaric acid | - b | TA a | 1.76 ± 0.08 | 0.71 ± 0.03 |
| 4 | Syringaldehyde | - b | 0.34 ± 0.02 | 0.57 ± 0.03 | 0.39 ± 0.02 |
| 5 | Luteolin | - b | - b | TA a | TA a |
| 6 | Apigenin | - b | - b | TA a | TA a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, W.-C.; Ling, X.-H.; Chang, C.-C.; Hsu, H.-F.; Wang, S.-W.; Lee, Y.-C.; Luo, C.; Lee, Y.-T.; Houng, J.-Y. Inhibitory Effects of Siegesbeckia orientalis Extracts on Advanced Glycation End Product Formation and Key Enzymes Related to Metabolic Syndrome. Molecules 2017, 22, 1785. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101785
Hung W-C, Ling X-H, Chang C-C, Hsu H-F, Wang S-W, Lee Y-C, Luo C, Lee Y-T, Houng J-Y. Inhibitory Effects of Siegesbeckia orientalis Extracts on Advanced Glycation End Product Formation and Key Enzymes Related to Metabolic Syndrome. Molecules. 2017; 22(10):1785. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101785
Chicago/Turabian StyleHung, Wei-Chin, Xue-Hua Ling, Chi-Chang Chang, Hsia-Fen Hsu, Shih-Wei Wang, Yi-Chen Lee, Ci Luo, Yun-Tzu Lee, and Jer-Yiing Houng. 2017. "Inhibitory Effects of Siegesbeckia orientalis Extracts on Advanced Glycation End Product Formation and Key Enzymes Related to Metabolic Syndrome" Molecules 22, no. 10: 1785. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22101785