UPLC Quantitative Analysis of Multi-Components by Single Marker and Quality Evaluation of Polygala tenuifolia Wild. Extracts
Abstract
:1. Introduction
2. Results and Discussion
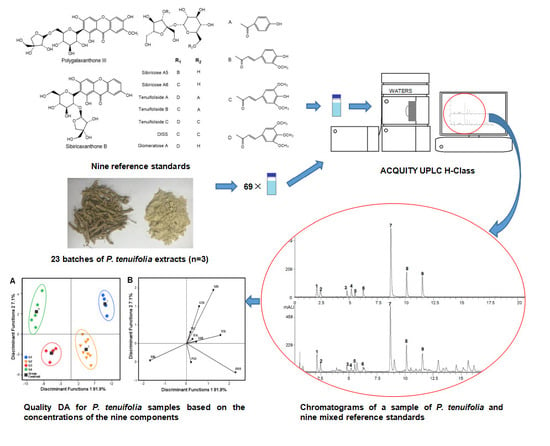
2.1. The Establishment of Analytical Protocol
2.1.1. Blank Control
2.1.2. Representative UPLC Chromatograms
2.1.3. The Building of Calibration Curves
2.1.4. Method Validation
2.1.5. Calculation of the Relative Calibration Factors
2.1.6. Robustness of the Relative Calibration Factors
2.1.7. Similarity Evaluation of the QAMS and ESM Methods
2.2. Quality Evaluation of P. tenuifolia Based on Nine Components
2.2.1. Concentrations of the Nine Components in the P. tenuifolia Samples
2.2.2. Hierarchical Clustering Analysis (HCA)
2.2.3. Radar Plot Analysis
2.2.4. Discrimination analysis (DA)
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Experimental Design
3.3. Chromatographic Conditions
3.4. Preparation of Sample Solutions
3.5. Preparation of Standard Solutions
3.6. Quantitative Analysis of Multi-Components by Single Marker
3.7. Assessment of the ESM and the QAMS Method
3.8. Evaluation and Calculation of Expanded Uncertainty
3.9. Data Analyses
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| QAMS | Quantitative analysis of multi-components by a single marker |
| TCM | Traditional Chinese medicine |
| SDMC | Simultaneous determination of multi-components |
| UPLC | Ultra-performance liquid chromatography |
| HPLC | High-performance liquid chromatography |
| ESM | External standard method |
| DISS | 3,6′-disinapoyl sucrose |
| RCFs | Relative calibration factors |
| HCA | Hierarchical cluster analysis |
| DA | Discriminant analysis |
| LOQ | Limit of quantification |
| LOD | Limit of detection |
| MDL | Method detection limit |
| MQL | Method quantitation limit |
| QA | Quality assurance |
| QC | Quality control |
| SD | Standard deviation |
References
- Xie, P.S.; Leung, A.Y.; Xie, P.S.; van Beek, T.A. Understanding the traditional aspect of Chinese medicine in order to achieve meaningful quality control of Chinese materia medica. J. Chromatogr. A 2009, 1216, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.H.; Qi, L.W.; Li, K.; Chu, C.; Li, P. Recent advances in quality control of traditional Chinese medicines. Comb. Chem. High Throughput Screen. 2010, 13, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liu, Z.; Liu, C.; Wu, M.; Su, H.; Ma, X.; Zang, Y.; Wang, J.; Zhao, Y.; Xiao, X. Spectrum-Effect Relationships Between Chemical Fingerprints and Antibacterial Effects of Lonicerae Japonicae Flos and Lonicerae Flos Base on UPLC and Microcalorimetry. Front. Pharmacol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Jakimska, A.; Kot-Wasik, A.; Namieśnik, J. The Current State-of-the-Art in the Determination of Pharmaceutical Residues in Environmental Matrices Using Hyphenated Techniques. Crit. Rev. Anal. Chem. 2014, 44, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Saini, G.; Nair, A.; Sharma, R. UPLC: A preeminent technique in pharmaceutical analysis. Acta Pol. Pharm. 2012, 69, 371–380. [Google Scholar] [PubMed]
- Sun, Z.; Zhao, Y.; Liu, T.; Sun, X.; Li, R.; Zhang, P.; Xiao, X. Spectrum-effect relationships between UPLC fingerprints and bioactivities of five Aconitum L. plants. Thermochim. Acta 2013, 558, 61–66. [Google Scholar] [CrossRef]
- Kong, W.J.; Zhao, Y.L.; Xiao, X.H.; Wang, J.B.; Li, H.B.; Li, Z.L.; Jin, C.; Liu, Y. Spectrum-effect relationships between ultra performance liquid chromatography fingerprints and anti-bacterial activities of Rhizoma coptidis. Anal. Chim. Acta 2009, 634, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.F.; Wu, T.; Nassar, S.F.; Wisnewski, A.V. UPLC-MS for metabolomics: A giant step forward in support of pharmaceutical research. Drug Discov. Today 2016, 22, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Q.; Ma, J.Q.; Ma, C.L.; Yao, M.Z.; Chen, L. Determination of catechin concentration in representative Chinese tea germplasms. J. Agric. Food Chem. 2014, 62, 9436–9441. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Jia, X.H.; Zhu, S.; Komatsu, K.; Wang, X.; Cai, S.Q. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta 2015, 134, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, Y.; Shao, W.; Gao, D. Analysis of the HPLC fingerprint and QAMS from Pyrrosia species. Ind. Crops Prod. 2016, 85, 29–37. [Google Scholar] [CrossRef]
- Du, Y.; Li, Q.; Liu, J.; Yin, Y.; Bi, K. Combinative method using multi-components quantitation by single reference standard and HPLC fingerprint for comprehensive evaluation of Rhodiola crenulata H.Ohba. Anal. Methods 2014, 6, 5891–5898. [Google Scholar] [CrossRef]
- Li, S.P.; Qiao, C.F.; Chen, Y.W.; Zhao, J.; Cui, X.M.; Zhang, Q.W.; Liu, X.M.; Hu, D.J. A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. J. Chromatogr. A 2013, 1313, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant Origin; Springer: Berlin/Heidelberg, Germany, 1992; pp. 997–1002. [Google Scholar]
- Dong, X.Z.; Huang, C.L.; Yu, B.Y.; Hu, Y.; Mu, L.H.; Liu, P. Effect of tenuifoliside A isolated from Polygala tenuifolia on the ERK and PI3K pathways in C6 glioma cells. Phytomedicine 2014, 21, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, T.; Li, L.; Zhao, Y.; Pei, L.; Zhao, J. Predicting the Potential Distribution of Polygala tenuifolia Willd. under Climate Change in China. PLoS ONE 2016, 11, e0163718. [Google Scholar] [CrossRef] [PubMed]
- Nagajyothi, P.C.; Sang, J.C.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Li, Z.; Xu, X.; Peng, B.; Qin, X.; Du, G. UPLC/Q-TOF MS-Based Metabolomics and qRT-PCR in Enzyme Gene Screening with Key Role in Triterpenoid Saponin Biosynthesis of Polygala tenuifolia. PLoS ONE 2014, 9, e105765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Song, X.; Li, L.; Wang, J.; Lin, L.; Li, C.; Li, H.; Lv, Y.; Jin, Y.; Liu, Y. Polygala tenuifolia polysaccharide PTP induced apoptosis in ovarian cancer cells via a mitochondrial pathway. Tumor Biol. 2015, 36, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, J.; Zhu, D.; Yu, B. Quality evaluation of Polygala japonica through simultaneous determination of six bioactive triterpenoid saponins by HPLC-ELSD. J. Pharm. Biomed. Anal. 2007, 43, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Zhang, L.J.; Liang, B.; Li Zhen, X.U.; Yang, S.L. Oligosaccharide esters isolated from plants of Polygalaceae. Chin. Tradit. Herb. Drugs 2002, 10, 954–958. [Google Scholar]
- Chen, S.L.; Lin, L.L.; Chen, S.B.; Yang, D.J.; Yang, J.S.; Xiao, P.G. Quantitative Determination of Nine Xanthones in Polygala caudata and Fingerprinting of Polygala L. by HPLC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2953–2963. [Google Scholar] [CrossRef]
- Sun, X.L.; Ito, H.; Masuoka, T.; Kamei, C.; Hatano, T. Effect of Polygala tenuifolia root extract on scopolamine-induced impairment of rat spatial cognition in an eight-arm radial maze task. Biol. Pharm. Bull. 2007, 30, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Liou, S.J.; Lee, T.H.; Chuang, Y.C.; Won, S.J. Xanthone derivatives as potential anti-cancer drugs. J. Pharm. Pharmacol. 1996, 48, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Chung, M.I.; Liou, S.J.; Lee, T.H.; Wang, J.P. Synthesis and anti-inflammatory effects of xanthone derivatives. J. Pharm. Pharmacol. 1996, 48, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, S.; Suwannapoch, N.; Phakhodee, W.; Thanuhiranlert, J.; Ratananukul, P.; Chimnoi, N.; Suksamrarn, A. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem. Pharm. Bull. 2003, 51, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.P.; Li, S.D.; Shi, Z.; Li, T.F.; Pan, R.L.; Chang, Q.; Qin, C.; Liu, X.M. Antidepressant-like effects and memory enhancement of a herbal formula in mice exposed to chronic mild stress. Sci. Bull. 2013, 29, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Chen, Y.; Liu, X.; Wang, Q.; Wang, L.; Jia, W.; Wang, Y. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, M.; Hui, X.; Huai, B.; Feng, W.; Pan, G.; Liao, X.; Liu, Y. Development of a modified QuEChERS method for the determination of veterinary antibiotics in swine manure by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2016, 1027, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.S.; Xue, Y.; Deng, W.L.; Liao, X.; Xu, X.M.; Li, B.G.; Liu, Y.M. Ligand fishing with functionalized magnetic nanoparticles coupled with mass spectrometry for herbal medicine analysis: Ligand fishing for herbal medicine analysis. Anal. Bioanal. Chem. 2011, 399, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Cheajesadagul, P.; Arnaudguilhem, C.; Shiowatana, J.; Siripinyanond, A.; Szpunar, J. Discrimination of geographical origin of rice based on multi-element fingerprinting by high resolution inductively coupled plasma mass spectrometry. Food Chem. 2013, 141, 3504–3509. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Song, Z.; Wang, Z.; Qian, Z.; Zhang, Q. Overview on quantitative analysis of multi-components by single-marker. China J. Chin. Mater. Med. 2012, 37, 405–416. [Google Scholar]
- Xie, J.; Li, J.; Liang, J.; Luo, P.; Qing, L.S.; Ding, L.S. Determination of concentrations of Catechins in Oolong Teas by Quantitative Analysis of Multi-components Via a Single Marker (QAMS) Method. Food Anal. Methods 2016, 10, 1–6. [Google Scholar] [CrossRef]
- International Organisation for Standardisation. Guide to the Expression of Uncertainty in Measurement (GUM); International Organisation for Standardisation: Geneva, Switzerland, 1993. [Google Scholar]
- Barwick, V.J.; Ellison, S.L.R. Measurement uncertainty: Approaches to the evaluation of uncertainties associated with recovery†. Analyst 1999, 124, 981–990. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Estimating uncertainty in analytical procedures based on chromatographic techniques. J. Chromatogr. A 2010, 1217, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Kannel, P.R.; Lee, S.; Kanel, S.R.; Khan, S.P. Chemometric application in classification and assessment of monitoring locations of an urban river system. Anal. Chim. Acta 2007, 582, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Saary, M.J. Radar plots: A useful way for presenting multivariate health care data. J. Clin. Epidemiol. 2008, 61, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Friendly, M.; Kwan, E. Effect ordering for data displays. Comput. Stat Data Anal. 2003, 43, 509–539. [Google Scholar] [CrossRef]
- Shi, X.M.; Zhang, J.S.; Tang, Q.J.; Yang, Y.; Hao, R.X.; Pan, Y.J. Fingerprint analysis of Lingzhi (Ganoderma) strains by high-performance liquid chromatography coupled with chemometric methods. World J. Microbiol. Biotechnol. 2008, 24, 2443–2450. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China, Part 1; China Medical Science Press: Beijing, China, 2015; pp. 156–157. ISBN 978-7-5067-7337-9. [Google Scholar]
- Zhao, Y.S.; Liu, X.; Mao, F.Y.; Tian, H.L.; Wan, D.G. Study on quality assessment of Polygalae Radix based on HPLC-DAD fingerprint. Zhongguo Zhong Yao Za Zhi 2014, 39, 3991–4000. [Google Scholar] [PubMed]
Sample Availability: Samples of the S1–S23 and the compounds 3,6′-disinapoyl sucrose (DISS), sibiricose A5, sibiricose A6, glomeratose A, tenuifoliside A, tenuifoliside B, tenuifoliside C, sibiricaxanthone B, and polygalaxanthone III are available from the authors. |







| Reference Substance | Rt | Regression Equation | R2 | Linear Range | LOD | LOQ | MDL | MQL |
|---|---|---|---|---|---|---|---|---|
| min | μg·mL−1 | μg·mL−1 | μg·mL−1 | μg·mL−1 | μg·mL−1 | |||
| sibiricose A5 | 1.99 | y = 6.606 × 106x + 1683.6 | 0.9998 | 6.71~93.90 | 0.14 | 0.45 | 0.08 | 0.26 |
| sibiricose A6 | 2.33 | y = 6.541 × 106x − 2614 | 0.9992 | 4.99~69.79 | 0.11 | 0.36 | 0.06 | 0.2 |
| sibiricaxanthone B | 4.72 | y = 4.412 × 106x − 545.72 | 0.9999 | 5.06~70.83 | 0.13 | 0.43 | 0.06 | 0.19 |
| glomeratose A | 5.11 | y = 5.450 × 106x − 839.47 | 0.9999 | 5.68~79.55 | 0.1 | 0.33 | 0.05 | 0.17 |
| polygalaxanthone III | 5.44 | y = 6.332 × 106x − 2350.7 | 0.9999 | 2.41~33.68 | 0.06 | 0.2 | 0.03 | 0.1 |
| tenuifoliside B | 6.19 | y = 4.907 × 106x − 1748.2 | 0.9999 | 5.72~80.12 | 0.2 | 0.66 | 0.08 | 0.26 |
| DISS | 8.69 | y = 9.222 × 106x – 24939 | 0.9999 | 34.20~478.83 | 0.06 | 0.22 | 0.04 | 0.12 |
| tenuifoliside A | 10.11 | y = 4.137 × 106x − 6735.5 | 0.9999 | 21.69~303.70 | 0.14 | 0.45 | 0.06 | 0.18 |
| tenuifoliside C | 11.54 | y = 9.104 × 106x − 5511.8 | 0.9999 | 9.03~126.42 | 0.06 | 0.21 | 0.03 | 0.11 |
| Compounds | Intra-Day RSD/% | Inter-Day RSD/% | Intermediate RSD/% | Stability RSD/% | Repeatability RSD/% | Recovery RSD/% |
|---|---|---|---|---|---|---|
| sibiricose A5 | 0.30 | 0.42 | 1.70 | 0.22 | 1.78 | 97.33, 1.05 |
| sibiricose A6 | 0.19 | 0.40 | 0.37 | 0.57 | 1.61 | 96.75, 2.36 |
| sibiricaxanthone B | 0.33 | 0.64 | 1.55 | 0.38 | 1.44 | 98.11, 1.03 |
| glomeratose A | 0.23 | 0.59 | 1.02 | 0.27 | 1.72 | 97.61, 1.03 |
| polygalaxanthone III | 0.37 | 0.50 | 1.40 | 0.29 | 1.34 | 97.93, 1.19 |
| tenuifoliside B | 0.69 | 0.48 | 0.62 | 1.40 | 1.76 | 101.90, 1.48 |
| DISS | 0.34 | 0.44 | 0.00 | 0.11 | 1.61 | 98.03, 1.08 |
| Tenuifoliside A | 0.60 | 0.65 | 0.75 | 0.21 | 3.27 | 97.22, 3.47 |
| tenuifoliside C | 0.83 | 1.07 | 1.85 | 2.86 | 1.68 | 95.48, 2.77 |
| No. | DISS | Sibiricose A5 | Sibiricose A6 | Sibiricaxanthone B | Glomeratose A | Polygalaxanthone III | Tenuifoliside B | Tenuifoliside A | Tenuifoliside C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QAMS | ESM | QASM | ESM | |
| S1 | 6.23 ± 0.25 | 1.45 ± 0.09 | 1.44 ± 0.07 | 1.08 ± 0.08 | 1.08 ± 0.07 | 0.96 ± 0.06 | 0.97 ± 0.05 | 0.64 ± 0.04 | 0.65 ± 0.03 | 0.46 ± 0.03 | 0.46 ± 0.02 | 0.48 ± 0.03 | 0.48 ± 0.03 | 4.03 ± 0.38 | 4.06 ± 0.35 | 1.82 ± 0.15 | 1.84 ± 0.13 |
| S2 | 7.15 ± 0.29 | 1.32 ± 0.08 | 1.31 ± 0.07 | 1.30 ± 0.10 | 1.30 ± 0.09 | 0.86 ± 0.05 | 0.87 ± 0.04 | 0.77 ± 0.05 | 0.77 ± 0.04 | 0.53 ± 0.04 | 0.54 ± 0.03 | 0.44 ± 0.03 | 0.45 ± 0.03 | 3.08 ± 0.29 | 3.12 ± 0.27 | 1.82 ± 0.15 | 1.84 ± 0.13 |
| S3 | 3.55 ± 0.16 | 0.57 ± 0.04 | 0.56 ± 0.03 | 0.52 ± 0.04 | 0.52 ± 0.04 | 0.66 ± 0.05 | 0.65 ± 0.04 | 0.88 ± 0.06 | 0.88 ± 0.04 | 0.55 ± 0.04 | 0.55 ± 0.03 | 0.31 ± 0.02 | 0.32 ± 0.02 | 3.68 ± 0.33 | 3.67 ± 0.29 | 1.88 ± 0.15 | 1.87 ± 0.12 |
| S4 | 4.89 ± 0.23 | 0.69 ± 0.05 | 0.68 ± 0.03 | 0.84 ± 0.07 | 0.84 ± 0.05 | 0.78 ± 0.06 | 0.78 ± 0.04 | 0.75 ± 0.05 | 0.75 ± 0.04 | 0.51 ± 0.04 | 0.51 ± 0.03 | 0.43 ± 0.03 | 0.44 ± 0.02 | 2.78 ± 0.27 | 2.81 ± 0.24 | 1.95 ± 0.16 | 1.96 ± 0.13 |
| S5 | 5.00 ± 0.23 | 1.03 ± 0.07 | 1.02 ± 0.05 | 1.03 ± 0.08 | 1.03 ± 0.07 | 0.70 ± 0.05 | 0.71 ± 0.04 | 0.69 ± 0.05 | 0.70 ± 0.04 | 0.50 ± 0.04 | 0.51 ± 0.03 | 0.32 ± 0.02 | 0.32 ± 0.02 | 2.62 ± 0.25 | 2.65 ± 0.22 | 1.51 ± 0.13 | 1.52 ± 0.11 |
| S6 | 6.95 ± 0.28 | 2.15 ± 0.15 | 2.13 ± 0.11 | 1.44 ± 0.12 | 1.44 ± 0.11 | 1.08 ± 0.07 | 1.09 ± 0.05 | 0.77 ± 0.05 | 0.78 ± 0.04 | 0.66 ± 0.04 | 0.66 ± 0.03 | 0.49 ± 0.03 | 0.49 ± 0.03 | 4.32 ± 0.38 | 4.36 ± 0.35 | 2.40 ± 0.20 | 2.41 ± 0.18 |
| S7 | 3.98 ± 0.16 | 1.33 ± 0.09 | 1.30 ± 0.06 | 1.04 ± 0.08 | 1.04 ± 0.07 | 0.81 ± 0.05 | 0.80 ± 0.04 | 0.88 ± 0.06 | 0.88 ± 0.04 | 0.53 ± 0.04 | 0.53 ± 0.03 | 0.31 ± 0.02 | 0.31 ± 0.02 | 4.32 ± 0.41 | 4.31 ± 0.37 | 1.82 ± 0.15 | 1.82 ± 0.13 |
| S8 | 5.16 ± 0.19 | 0.98 ± 0.06 | 0.97 ± 0.05 | 1.00 ± 0.07 | 1.00 ± 0.06 | 0.76 ± 0.05 | 0.77 ± 0.04 | 0.78 ± 0.05 | 0.78 ± 0.04 | 0.51 ± 0.03 | 0.51 ± 0.03 | 0.43 ± 0.03 | 0.44 ± 0.02 | 2.65 ± 0.23 | 2.68 ± 0.21 | 1.17 ± 0.09 | 1.18 ± 0.08 |
| S9 | 7.01 ± 0.29 | 1.38 ± 0.10 | 1.38 ± 0.08 | 1.38 ± 0.11 | 1.38 ± 0.09 | 0.65 ± 0.05 | 0.66 ± 0.04 | 0.81 ± 0.06 | 0.82 ± 0.05 | 0.49 ± 0.04 | 0.50 ± 0.03 | 0.55 ± 0.04 | 0.55 ± 0.04 | 2.39 ± 0.23 | 2.43 ± 0.21 | 2.00 ± 0.17 | 2.02 ± 0.15 |
| S10 | 6.45 ± 0.31 | 1.87 ± 0.13 | 1.86 ± 0.09 | 1.77 ± 0.15 | 1.76 ± 0.12 | 1.36 ± 0.10 | 1.36 ± 0.08 | 1.11 ± 0.07 | 1.12 ± 0.05 | 0.73 ± 0.05 | 0.73 ± 0.04 | 0.63 ± 0.05 | 0.64 ± 0.04 | 5.09 ± 0.48 | 5.12 ± 0.42 | 2.29 ± 0.19 | 2.30 ± 0.16 |
| S11 | 5.89 ± 0.24 | 0.80 ± 0.05 | 0.79 ± 0.04 | 0.91 ± 0.07 | 0.91 ± 0.06 | 0.86 ± 0.05 | 0.86 ± 0.04 | 0.96 ± 0.06 | 0.97 ± 0.05 | 0.54 ± 0.04 | 0.55 ± 0.03 | 0.54 ± 0.04 | 0.55 ± 0.03 | 3.33 ± 0.32 | 3.36 ± 0.29 | 1.43 ± 0.11 | 1.44 ± 0.10 |
| S12 | 5.46 ± 0.25 | 1.91 ± 0.12 | 1.89 ± 0.09 | 1.37 ± 0.11 | 1.36 ± 0.09 | 1.16 ± 0.08 | 1.17 ± 0.05 | 0.86 ± 0.05 | 0.86 ± 0.04 | 0.88 ± 0.06 | 0.88 ± 0.04 | 0.93 ± 0.06 | 0.93 ± 0.05 | 5.17 ± 0.47 | 5.19 ± 0.41 | 2.01 ± 0.16 | 2.02 ± 0.13 |
| S13 | 6.59 ± 0.27 | 1.45 ± 0.09 | 1.44 ± 0.07 | 1.59 ± 0.12 | 1.59 ± 0.10 | 0.83 ± 0.06 | 0.84 ± 0.05 | 0.72 ± 0.04 | 0.73 ± 0.03 | 0.55 ± 0.04 | 0.56 ± 0.03 | 0.61 ± 0.04 | 0.61 ± 0.04 | 4.20 ± 0.40 | 4.24 ± 0.36 | 2.14 ± 0.17 | 2.16 ± 0.14 |
| S14 | 4.96 ± 0.19 | 0.73 ± 0.05 | 0.71 ± 0.04 | 0.85 ± 0.07 | 0.85 ± 0.06 | 0.74 ± 0.04 | 0.74 ± 0.04 | 0.68 ± 0.04 | 0.68 ± 0.03 | 0.48 ± 0.03 | 0.49 ± 0.03 | 0.41 ± 0.03 | 0.41 ± 0.02 | 2.69 ± 0.25 | 2.71 ± 0.23 | 1.31 ± 0.11 | 1.32 ± 0.10 |
| S15 | 3.74 ± 0.16 | 0.79 ± 0.05 | 0.78 ± 0.04 | 0.75 ± 0.06 | 0.75 ± 0.05 | 0.77 ± 0.05 | 0.76 ± 0.04 | 0.94 ± 0.07 | 0.94 ± 0.06 | 0.48 ± 0.03 | 0.48 ± 0.02 | 0.33 ± 0.02 | 0.33 ± 0.02 | 3.74 ± 0.34 | 3.73 ± 0.30 | 1.50 ± 0.13 | 1.50 ± 0.11 |
| S16 | 6.70 ± 0.30 | 2.22 ± 0.14 | 2.21 ± 0.10 | 1.48 ± 0.12 | 1.47 ± 0.10 | 1.11 ± 0.07 | 1.12 ± 0.06 | 0.80 ± 0.05 | 0.81 ± 0.04 | 0.67 ± 0.05 | 0.67 ± 0.04 | 0.53 ± 0.04 | 0.54 ± 0.04 | 4.51 ± 0.42 | 4.54 ± 0.37 | 2.30 ± 0.18 | 2.32 ± 0.15 |
| S17 | 4.19 ± 0.21 | 0.96 ± 0.07 | 0.94 ± 0.04 | 0.94 ± 0.08 | 0.94 ± 0.06 | 0.71 ± 0.05 | 0.71 ± 0.03 | 0.78 ± 0.06 | 0.78 ± 0.04 | 0.46 ± 0.03 | 0.46 ± 0.02 | 0.33 ± 0.03 | 0.34 ± 0.02 | 2.73 ± 0.27 | 2.75 ± 0.24 | 1.38 ± 0.12 | 1.38 ± 0.09 |
| S18 | 3.56 ± 0.17 | 1.02 ± 0.07 | 1.00 ± 0.05 | 0.70 ± 0.06 | 0.70 ± 0.05 | 0.69 ± 0.05 | 0.69 ± 0.03 | 0.69 ± 0.05 | 0.69 ± 0.04 | 0.53 ± 0.04 | 0.53 ± 0.03 | 0.31 ± 0.03 | 0.32 ± 0.02 | 3.15 ± 0.30 | 3.15 ± 0.26 | 1.39 ± 0.12 | 1.39 ± 0.10 |
| S19 | 5.20 ± 0.25 | 1.19 ± 0.08 | 1.18 ± 0.06 | 1.11 ± 0.10 | 1.11 ± 0.08 | 0.73 ± 0.05 | 0.73 ± 0.04 | 0.62 ± 0.04 | 0.63 ± 0.03 | 0.47 ± 0.03 | 0.47 ± 0.03 | 0.42 ± 0.03 | 0.44 ± 0.03 | 2.47 ± 0.24 | 2.50 ± 0.21 | 1.34 ± 0.11 | 1.35 ± 0.09 |
| S20 | 6.11 ± 0.23 | 1.60 ± 0.10 | 1.58 ± 0.08 | 1.10 ± 0.08 | 1.10 ± 0.07 | 0.93 ± 0.05 | 0.94 ± 0.04 | 0.71 ± 0.04 | 0.72 ± 0.03 | 0.74 ± 0.05 | 0.74 ± 0.04 | 0.46 ± 0.03 | 0.48 ± 0.03 | 3.49 ± 0.33 | 3.53 ± 0.31 | 2.26 ± 0.17 | 2.27 ± 0.15 |
| S21 | 5.71 ± 0.21 | 1.16 ± 0.07 | 1.15 ± 0.05 | 1.22 ± 0.09 | 1.22 ± 0.08 | 1.08 ± 0.06 | 1.09 ± 0.05 | 0.78 ± 0.05 | 0.78 ± 0.04 | 0.77 ± 0.05 | 0.77 ± 0.04 | 0.85 ± 0.05 | 0.88 ± 0.04 | 4.08 ± 0.35 | 4.10 ± 0.32 | 1.32 ± 0.10 | 1.33 ± 0.09 |
| S22 | 7.04 ± 0.29 | 1.66 ± 0.11 | 1.65 ± 0.08 | 1.48 ± 0.12 | 1.48 ± 0.10 | 0.89 ± 0.06 | 0.89 ± 0.04 | 0.76 ± 0.05 | 0.77 ± 0.04 | 0.61 ± 0.04 | 0.61 ± 0.03 | 0.35 ± 0.03 | 0.35 ± 0.02 | 3.43 ± 0.33 | 3.47 ± 0.30 | 1.66 ± 0.13 | 1.67 ± 0.12 |
| S23 | 6.35 ± 0.29 | 1.87 ± 0.12 | 1.86 ± 0.08 | 1.33 ± 0.11 | 1.33 ± 0.09 | 0.86 ± 0.07 | 0.86 ± 0.05 | 0.70 ± 0.05 | 0.70 ± 0.03 | 0.61 ± 0.05 | 0.62 ± 0.04 | 0.40 ± 0.03 | 0.41 ± 0.02 | 3.11 ± 0.29 | 3.14 ± 0.26 | 1.83 ± 0.15 | 1.85 ± 0.12 |
| Mean | 5.523 | 1.285 | 1.271 | 1.140 | 1.139 | 0.867 | 0.872 | 0.786 | 0.789 | 0.577 | 0.578 | 0.471 | 0.480 | 3.524 | 3.548 | 1.762 | 1.772 |
| Cir | 0.999990 | 0.999998 | 0.999994 | 0.999990 | 0.999993 | 0.999920 | 0.999990 | 0.999993 | |||||||||
| Group | Assigned Origin for All of the Cultivated P. tenuifolia Samples with Nine Components | |||||
| G1 | G2 | G3 | G4 | Total | Original Correct (%) | |
| G1 | 4 | 0 | 0 | 0 | 4 | 100.0 |
| G2 | 0 | 9 | 0 | 0 | 9 | 100.0 |
| G3 | 0 | 0 | 5 | 0 | 5 | 100.0 |
| G4 | 0 | 0 | 0 | 5 | 5 | 100.0 |
| Total | 4 | 9 | 5 | 5 | 23 | 100.0 |
| Group | G1 | G2 | G3 | G4 | Total | Cross-Validation Correct (%) |
| G1 | 4 | 0 | 0 | 0 | 4 | 100.0 |
| G2 | 0 | 9 | 0 | 0 | 9 | 100.0 |
| G3 | 0 | 0 | 5 | 0 | 5 | 100.0 |
| G4 | 0 | 0 | 0 | 5 | 5 | 100.0 |
| Total | 4 | 9 | 5 | 5 | 23 | 100.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Mao, F.; Zhao, Y.; Wang, W.; Fan, L.; Gao, X.; Zhao, J.; Tian, H. UPLC Quantitative Analysis of Multi-Components by Single Marker and Quality Evaluation of Polygala tenuifolia Wild. Extracts. Molecules 2017, 22, 2276. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122276
Xu R, Mao F, Zhao Y, Wang W, Fan L, Gao X, Zhao J, Tian H. UPLC Quantitative Analysis of Multi-Components by Single Marker and Quality Evaluation of Polygala tenuifolia Wild. Extracts. Molecules. 2017; 22(12):2276. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122276
Chicago/Turabian StyleXu, Rui, Fuying Mao, Yunsheng Zhao, Wenping Wang, Lingling Fan, Xiaojuan Gao, Jianjun Zhao, and Hongling Tian. 2017. "UPLC Quantitative Analysis of Multi-Components by Single Marker and Quality Evaluation of Polygala tenuifolia Wild. Extracts" Molecules 22, no. 12: 2276. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22122276