How and How Much Molecular Conformation Affects Electronic Circular Dichroism: The Case of 1,1-Diarylcarbinols
Abstract
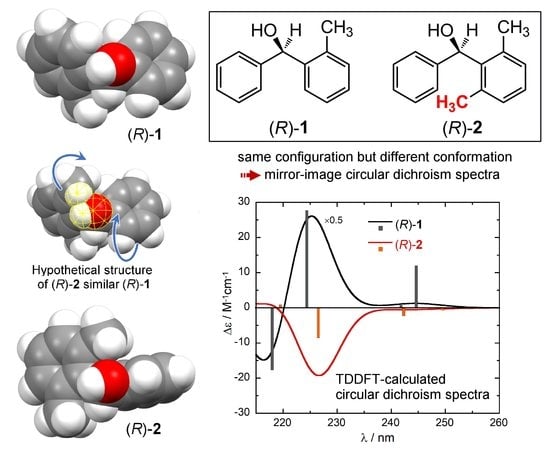
:1. Introduction
2. Results
2.1. Experimental and Calculated ECD Spectra
2.2. Vibronic ECD Calculations (1Lb Band)
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, P.L. Chiroptical Spectroscopy: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pescitelli, G.; Di Bari, L.; Berova, N. Conformational Aspects in the Studies of Organic Compounds by Electronic Circular Dichroism. Chem. Soc. Rev. 2011, 40, 4603–4625. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, A.; Casarini, D. Recent trends in conformational analysis. WIREs Comput. Mol. Sci. 2012, 2, 613–641. [Google Scholar] [CrossRef]
- Pescitelli, G.; Di Bari, L.; Berova, N. Application of electronic circular dichroism in the study of supramolecular systems. Chem. Soc. Rev. 2014, 43, 5211–5233. [Google Scholar] [CrossRef] [PubMed]
- Zajac, G.; Kaczor, A.; Buda, S.; Młynarski, J.; Frelek, J.; Dobrowolski, J.C.; Baranska, M. Prediction of ROA and ECD Related to Conformational Changes of Astaxanthin Enantiomers. J. Phys. Chem. B 2015, 119, 12193–12201. [Google Scholar] [CrossRef] [PubMed]
- Masnyk, M.; Butkiewicz, A.; Górecki, M.; Luboradzki, R.; Bannwarth, C.; Grimme, S.; Frelek, J. Synthesis and Comprehensive Structural and Chiroptical Characterization of Enones Derived from (−)-α-Santonin by Experiment and Theory. J. Org. Chem. 2016, 81, 4588–4600. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Goto, H. Theoretical Electronic Circular Dichroism Study of 1,3-Diene Derivatives for the Elucidation of ECD Spectra of 1,3-Cyclohexadiene and Its Derivatives. Chirality 2015, 27, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, H.; Spinello, A.; Terenzi, A.; Assfeld, X.; Barone, G.; Monari, A. Circular Dichroism of DNA G-Quadruplexes: Combining Modeling and Spectroscopy To Unravel Complex Structures. J. Phys. Chem. B 2016, 120, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, C.; Seibert, J.; Grimme, S. Electronic Circular Dichroism of [16]Helicene With Simplified TD-DFT: Beyond the Single Structure Approach. Chirality 2016, 28, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Molteni, E.; Onida, G.; Tiana, G. Conformational Dependence of the Circular Dichroism Spectra of Single Amino Acids from Plane-Waves-Based Density Functional Theory Calculations. J. Phys. Chem. B 2015, 119, 4803–4811. [Google Scholar] [CrossRef] [PubMed]
- Štepánek, P.; Bouř, P. Multi-scale modeling of electronic spectra of three aromatic amino acids: Importance of conformational averaging and explicit solute-solvent interactions. Phys. Chem. Chem. Phys. 2014, 16, 20639–20649. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, T.; Nakatsuji, H. Conformational Dependence of the Circular Dichroism Spectrum of α-Hydroxyphenylacetic Acid: A ChiraSac Study. J. Phys. Chem. A 2013, 117, 14065–14074. [Google Scholar] [CrossRef] [PubMed]
- Ścianowski, J.; Pacuła, A.J.; Skowronek, P. Chiral dialkyl ditellurides: Conformation of chiral chromophore by circular dichroism and DFT calculation. Chirality 2017, 29, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, R.F.; Thomasi, S.S.; Ferreira, A.G.; Cass, Q.B.; Batista Junior, J.M. Solution-state conformations of natural products from chiroptical spectroscopy: The case of isocorilagin. Org. Biomol. Chem. 2016, 14, 3369–3375. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Bruhn, T. Comment on “Cocaine Hydrochloride Structure in Solution Revealed by Three Chiroptical Methods”. ChemPhysChem 2017, 18, 2549–2551. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Pescitelli, G.; Witterauf, F.; Ahrens, J.; Funk, M.; Wolfram, B.; Schneider, H.; Radius, U.; Bröring, M. Cryptochirality in 2,2′-Coupled BODIPY DYEmers. Eur. J. Org. Chem. 2016, 2016, 4236–4243. [Google Scholar] [CrossRef]
- Padula, D.; Lahoz, I.R.; Díaz, C.; Hernández, F.E.; Di Bari, L.; Rizzo, A.; Santoro, F.; Cid, M.M. A Combined Experimental–Computational Investigation to Uncover the Puzzling (Chiro-)optical Response of Pyridocyclophanes: One- and Two-Photon Spectra. Chem. Eur. J. 2015, 21, 12136–12147. [Google Scholar] [CrossRef] [PubMed]
- Padula, D.; Jurinovich, S.; Di Bari, L.; Mennucci, B. Simulation of Electronic Circular Dichroism of Nucleic Acids: From the Structure to the Spectrum. Chem. Eur. J. 2016, 22, 17011–17019. [Google Scholar] [CrossRef] [PubMed]
- Gropp, C.; Trapp, N.; Diederich, F. Alleno-Acetylenic Cage (AAC) Receptors: Chiroptical Switching and Enantioselective Complexation of trans-1,2-Dimethylcyclohexane in a Diaxial Conformation. Angew. Chem. Int. Ed. 2016, 55, 14444–14449. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Du, G.; Liu, K.; Jiang, L.; Ling, J.; Shen, Z. Chiroptical Inversion Induced by Rotation of a Carbon–Carbon Single Bond: An Experimental and Theoretical Study. J. Phys. Chem. A 2014, 118, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Isla, H.; Srebro-Hooper, M.; Jean, M.; Vanthuyne, N.; Roisnel, T.; Lunkley, J.L.; Muller, G.; Williams, J.A.G.; Autschbach, J.; Crassous, J. Conformational changes and chiroptical switching of enantiopure bis-helicenic terpyridine upon Zn2+ binding. Chem. Commun. 2016, 52, 5932–5935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennartson, A.; Hedström, A.; Håkansson, M. Spontaneous Generation of Chirality in Simple Diaryl Ethers. Chirality 2015, 27, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.; Choi, C.M.; Eun, H.J.; Jeong, C.; Heo, J.; Kim, N.J. Conformation-Specific Circular Dichroism Spectroscopy of Cold, Isolated Chiral Molecules. Angew. Chem. Int. Ed. 2014, 53, 7805–7808. [Google Scholar] [CrossRef] [PubMed]
- Daly, S.; Tia, M.; Garcia, G.A.; Nahon, L.; Powis, I. The Interplay between Conformation and Absolute Configuration in Chiral Electron Dynamics of Small Diols. Angew. Chem. Int. Ed. 2016, 55, 11054–11058. [Google Scholar] [CrossRef] [PubMed]
- Ripa, L.; Hallberg, A.; Sandström, J. Determination of Absolute Configurations of N-Formyl-3,3′,4,4′-tetrahydrospiro[naphthalene-1(2H),2′(1′H)-pyridine] (2) and N-Formyl-3′,4′-dihydrospiro[indan-1,2′(1′H)-pyridine] (3) by Analysis of Circular Dichroism Spectra. A Case of Two Compounds with Similar Configuration but Nearly Mirror Image CD Spectra. J. Am. Chem. Soc. 1997, 119, 5701–5705. [Google Scholar] [CrossRef]
- Padula, D.; Di Bari, L.; Pescitelli, G. The “Case of Two Compounds with Similar Configuration but Nearly Mirror Image CD Spectra” Refuted. Reassignment of the Absolute Configuration of N-Formyl-3′,4′-dihydrospiro indan-1,2′(1′H)-pyridine. J. Org. Chem. 2016, 81, 7725–7732. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, M.; Kuwahara, S.; Watanabe, M.; Harada, N.; Job, G.E.; Pirkle, W.H. Comparison of CD Spectra of (2-Methylphenyl)- and (2,6-Dimethylphenyl)-phenylmethanols Leads to Erroneous Absolute Configurations. Enantiomer 2002, 7, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Koumura, N.; Feringa, B.L. Chemistry of Unique Chiral Olefins. 3. Synthesis and Absolute Stereochemistry of trans- and cis-1,1′,2,2′,3,3′,4,4′-Octahydro-3,3′-dimethyl-4,4′-biphenanthrylidenes. J. Am. Chem. Soc. 1997, 119, 7256–7264. [Google Scholar] [CrossRef]
- Harada, N. HPLC Separation of Diastereomers: Chiral Molecular tools useful for the preparation of enantiopure compounds and simultaneous determination of their absolute configurations. Molecules 2016, 21, 1328. [Google Scholar] [CrossRef] [PubMed]
- Harada, N. Powerful chiral molecular tools for preparation of enantiopure alcohols and simultaneous determination of their absolute configurations by X-ray crystallography and/or 1H NMR anisotropy methods. In Chirality in Drug Research; Francotte, E., Lindnder, W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 283–321. [Google Scholar]
- Harada, N. Determination of absolute configurations by X-ray crystallography and 1H NMR anisotropy. Chirality 2008, 20, 691–723. [Google Scholar] [CrossRef] [PubMed]
- Naito, J.; Yamamoto, Y.; Akagi, M.; Sekiguchi, S.; Watanabe, M.; Harada, N. Unambiguous Determination of the Absolute Configurations of Acetylene Alcohols by Combination of the Sonogashira Reaction and the CD Exciton Chirality Method—Exciton Coupling between Phenylacetylene and Benzoate Chromophores. Monatsh. Chem. 2005, 136, 411–445. [Google Scholar] [CrossRef]
- Harada, N. Determination of Absolute Configurations by Electronic CD Exciton Chirality, Vibrational CD, 1H NMR Anisotropy, and X-ray Crystallography Methods-Principles, Practices, and Reliability. In Structure Elucidation in Organic Chemistry: The Search for the Right Tools; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 393–444. [Google Scholar]
- Padula, D.; Cerezo, J.; Pescitelli, G.; Santoro, F. The shape of the electronic circular dichroism spectrum of (2,6-dimethylphenyl)(phenyl)methanol: Interplay between conformational equilibria and vibronic effects. Phys. Chem. Chem. Phys. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jaffé, H.H.; Orchin, M. Theory and Applications of Ultraviolet Spectroscopy; Wiley: New York, NY, USA, 1962. [Google Scholar]
- Herzberg, G. Molecular Spectra and Molecular Structure. III. Electronic Spectra and Electronic Structure of Polyatomic Molecules; D. Van Nostrand Co.: Princeton, NI, USA, 1966. [Google Scholar]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Srebro-Hooper, M.; Autschbach, J. Calculating Natural Optical Activity of Molecules from First Principles. Annu. Rev. Phys. Chem. 2017, 68, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Spartan’16; v. 16.2.1; Wavefuntion, Inc.: Irvine, CA, USA, 2017.
- Mennucci, B.; Cammi, R. Continuum Solvation Models in Chemical Physics: From Theory to Applications; Wiley: Chichester, UK, 2007. [Google Scholar]
- Jacquemin, D.; Perpète, E.A.; Scuseria, G.E.; Ciofini, I.; Adamo, C. TD-DFT Performance for the Visible Absorption Spectra of Organic Dyes: Conventional versus Long-Range Hybrids. J. Chem. Theory Comput. 2008, 4, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Grimme, S.; Parac, M. Substantial Errors from Time-Dependent Density Functional Theory for the Calculation of Excited States of Large π Systems. ChemPhysChem 2003, 4, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Parac, M.; Grimme, S. A TDDFT study of the lowest excitation energies of polycyclic aromatic hydrocarbons. Chem. Phys. 2003, 292, 11–21. [Google Scholar] [CrossRef]
- Pescitelli, G.; Barone, V.; Di Bari, L.; Rizzo, A.; Santoro, F. Vibronic coupling dominates the electronic circular dichroism of the benzene chromophore 1Lb band. J. Org. Chem. 2013, 78, 7398–7405. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Di Bari, L.; Caporusso, A.M.; Salvadori, P. The prediction of the circular dichroism of the benzene chromophore: TDDFT calculations and sector rules. Chirality 2008, 20, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Willis, T.C. Optically active amines. XI. Optical rotatory dispersion and circular dichroism observations on α- and β-phenylalkylamine hydrochlorides. J. Am. Chem. Soc. 1971, 93, 2282–2290. [Google Scholar] [CrossRef]
- Horwitz, J.; Strickland, E.H.; Billups, C. Analysis of vibrational structure in the near-ultraviolet circular dichroism and absorption spectra of phenylalanine and its derivatives. J. Am. Chem. Soc. 1969, 91, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Avila Ferrer, F.J.; Santoro, F. Comparison of vertical and adiabatic harmonic approaches for the calculation of the vibrational structure of electronic spectra. Phys. Chem. Chem. Phys. 2012, 14, 13549–13563. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Cerezo, J. FCclasses, a Fortran 77 Code. Available online: http://www.pi.iccom.cnr.it/fcclasses (accessed on 8 January 2018).
- Cerezo, J.; Santoro, F. Revisiting Vertical Models to Simulate the Line Shape of Electronic Spectra Adopting Cartesian and Internal Coordinates. J. Chem. Theory Comput. 2016, 12, 4970–4985. [Google Scholar] [CrossRef] [PubMed]
- Petruska, J. Changes in the Electronic Transitions of Aromatic Hydrocarbons on Chemical Substitution. II. Application of Perturbation Theory to Substituted-Benzene Spectra. J. Chem. Phys. 1961, 34, 1120–1136. [Google Scholar] [CrossRef]
- Platt, J.R. Spectroscopic Moment: A Parameter of Substituent Groups Determining Aromatic Ultraviolet Intensities. J. Chem. Phys. 1951, 19, 263–271. [Google Scholar] [CrossRef]
- Andraud, C.; Garcìa, C.; Collet, A. S-Substituted Aromatics and Exciton Chirality. In Circular Dichroism: Principles and Applications, 2nd ed.; Berova, N., Nakanishi, K., Woody, R.W., Eds.; John Wiley & Sons: New York, NY, USA, 2000; pp. 305–336. [Google Scholar]
- Di Bari, L.; Pescitelli, G.; Marchetti, F.; Salvadori, P. Anomalous CD/UV Exciton Splitting of a Binaphthyl Derivative: The Case of 2,2′-Diiodo-1,1′-binaphthalene. J. Am. Chem. Soc. 2000, 122, 6395–6398. [Google Scholar] [CrossRef]
- Snatzke, G. Circular Dichroism and Absolute Conformation: Application of Qualitative MO Theory to Chiroptical Phenomena. Angew. Chem. Int. Ed. Eng. 1979, 18, 363–377. [Google Scholar] [CrossRef]
- Mason, S.M. Molecular Optical Activity & the Chiral Discriminations; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Harada, N.; Nakanishi, K. Circular Dichroic Spectroscopy—Exciton Coupling in Organic Stereochemistry; University Science Books: Mill Valley, CA, USA, 1983. [Google Scholar]
- Kwit, M.; Skowronek, P.; Gawronski, J.; Frelek, J.; Woznica, M.; Butkiewicz, A. Some Inherently Chiral Chromophores—Empirical Rules and Quantum Chemical Calculations. In Comprehensive Chiroptical Spectroscopy; Berova, N., Polavarapu, P.L., Nakanishi, K., Woody, R.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 2, pp. 37–72. [Google Scholar]
- Kurtán, T.; Antus, S.; Pescitelli, G. Electronic CD of Benzene and other Aromatic Chromophores for Determination of Absolute Configuration. In Comprehensive Chiroptical Spectroscopy; Berova, N., Polavarapu, P.L., Nakanishi, K., Woody, R.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 2, pp. 73–114. [Google Scholar]
- DeAngelis, G.G.; Wildman, W.C. Circular dichroism studies—I: A quadrant rule for the optically active aromatic chromophore in rigid polycyclic systems. Tetrahedron 1969, 25, 5099–5112. [Google Scholar] [CrossRef]
- Snatzke, G.; Ho, P.C. Circular dichroism—XLVI: Rules for benzene cotton-effects. Tetrahedron 1971, 27, 3645–3653. [Google Scholar] [CrossRef]
- Smith, H.E. Chiroptical Properties of the Benzene Chromophore. A Method for the Determination of the Absolute Configurations of Benzene Compounds by Application of the Benzene Sector and Benzene Chirality Rules. Chem. Rev. 1998, 98, 1709–1740. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Pescitelli, G. SpecDis Version 1.71. Available online: https://specdis-software.jimdo.com/ (accessed on 8 January 2018).
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Avila Ferrer, F.J.; Cerezo, J.; Soto, J.; Improta, R.; Santoro, F. First-principle computation of absorption and fluorescence spectra in solution accounting for vibronic structure, temperature effects and solvent inhomogenous broadening. Comput. Theor. Chem. 2014, 1040, 328–337. [Google Scholar] [CrossRef]
Sample Availability: Not available. |









| (R)-1 | (R)-2 | |||||
|---|---|---|---|---|---|---|
| Conf. 1 | Energy 2 | Pop. 3 | ϕ 4 | Energy 2 | Pop. 3 | ϕ 4 |
| #1 | 0 | 41.0 | 72.5 | 0 | 52.3 | −61.6 |
| #2 | 0.139 | 32.4 | 74.5 | 0.366 | 28.3 | −70.7 |
| #3 | 0.748 | 11.7 | −60.4 | 0.592 | 19.4 | −60.8 |
| #4 | 0.996 | 7.69 | 156.3 | |||
| #5 | 1.444 | 3.62 | −66.8 | |||
| #6 | 1.451 | 3.59 | −61.7 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padula, D.; Pescitelli, G. How and How Much Molecular Conformation Affects Electronic Circular Dichroism: The Case of 1,1-Diarylcarbinols. Molecules 2018, 23, 128. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23010128
Padula D, Pescitelli G. How and How Much Molecular Conformation Affects Electronic Circular Dichroism: The Case of 1,1-Diarylcarbinols. Molecules. 2018; 23(1):128. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23010128
Chicago/Turabian StylePadula, Daniele, and Gennaro Pescitelli. 2018. "How and How Much Molecular Conformation Affects Electronic Circular Dichroism: The Case of 1,1-Diarylcarbinols" Molecules 23, no. 1: 128. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23010128