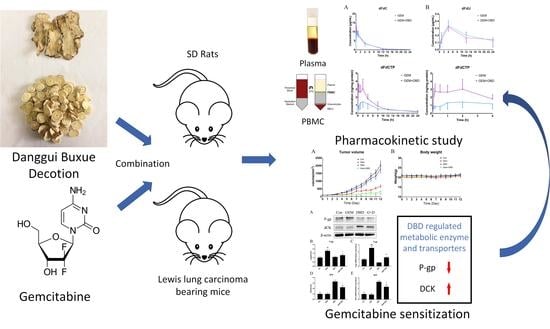
Danggui Buxue Decoction Sensitizes the Response of Non-Small-Cell Lung Cancer to Gemcitabine via Regulating Deoxycytidine Kinase and P-glycoprotein
Abstract
:1. Introduction
2. Results
2.1. Determination of the Combination Effect of DBD and GEM
2.2. In Vivo Plasma Comparative Pharmacokinetics
2.3. In Vivo Peripheral Blood Mononuclear Cells (PBMC) Comparative Pharmacokinetics
2.4. Protein Expression of dCK and P-gp in Lewis Lung Carcinoma (LLC)-bearing Mice Tumor Tissue
2.5. mRNA Expression of dCK and P-gp in Lewis Lung Carcinoma (LLC)-bearing Mice Tumor Tissue
2.6. Immune Regulatory Effect of DBD on LLC-Bearing Mice
2.7. P-gp Efflux Activity Measurement by Rh 123 Accumulation Assay
2.8. Protein Expression of P-gp in DBD Treated A549 Cells
2.9. mRNA Expression of hENT1 and hCNT1 in DBD Treated A549 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Preparation and Determination of DBD
4.3. Cell Culture
4.4. LLC Tumor Model
4.5. Western-Blot Analysis
4.6. Quantitative Polymerase Chain Reaction (qPCR)
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Rhodamine 123 (Rh 123) Accumulation Analysis
4.9. Pharmacokinetic Study in Rats
4.10. LC-MS/MS Based Bioanalytical Assays
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’Amico, T.A.; DeCamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr Cancer Netw. 2017, 15, 504–535. [Google Scholar] [CrossRef]
- Mackey, J.R.; Mani, R.S.; Selner, M.; Mowles, D.; Young, J.D.; Belt, J.A.; Crawford, C.R.; Cass, C.E. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998, 58, 4349–4357. [Google Scholar]
- Ritzel, M.W.; Ng, A.M.; Yao, S.Y.; Graham, K.; Loewen, S.K.; Smith, K.M.; Hyde, R.J.; Karpinski, E.; Cass, C.E.; Baldwin, S.A.; et al. Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: Identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). Mol. Membr. Biol. 2001, 18, 65–72. [Google Scholar] [PubMed]
- Wong, A.; Soo, R.A.; Yong, W.P.; Innocenti, F. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug MeTab. Rev. 2009, 41, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Mini, E.; Nobili, S.; Caciagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17 (Suppl. 5), v7–v12. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A., Jr. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, M.; Qiu, X.; Lu, Y. The potential of herb medicines in the treatment of esophageal cancer. Biomed. Pharm. 2018, 103, 381–390. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Li, J.; He, L.; Tripathy, D. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane. Database Syst. Rev. 2007, 2, CD004921. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jia, X.; Liu, J.P.; Liao, J.; Yang, Y. Herbal medicines for advanced colorectal cancer. Cochrane. Database Syst. Rev. 2012, 5, CD004653. [Google Scholar]
- Engdal, S.; Klepp, O.; Nilsen, O.G. Identification and exploration of herb-drug combinations used by cancer patients. Integr. Cancer 2009, 8, 29–36. [Google Scholar] [CrossRef]
- Liu, C.X.; Yi, X.L.; Si, D.Y.; Xiao, X.F.; He, X.; Li, Y.Z. Herb-drug interactions involving drug metabolizing enzymes and transporters. Curr. Drug MeTab. 2011, 12, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Coley, H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control 2003, 10, 159–165. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Liu, D.L.; Li, H.; Luo, J.; Zhang, J.H.; Li, Y.; Chen, K.J.; Tong, H.F.; Lin, S.Z. Emodin sensitizes the gemcitabine-resistant cell line Bxpc-3/Gem to gemcitabine via downregulation of NF-kappaB and its regulated targets. Int. J. Onco. 2013, 42, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, G.; Gong, Y.; Yu, Q.; He, B.; Li, W.; He, Z.; Hao, W.; He, Z.; Liu, Y. Silencing of TRPM8 inhibits aggressive tumor phenotypes and enhances gemcitabine sensitivity in pancreatic cancer. Pancreatology 2018, 18, 935–944. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, M.; Xu, S.W.; Chen, W.; Long, M.M.; Shi, Y.H.; Liu, Q.; Mohan, M.; Wang, J. miR-218-5p restores sensitivity to gemcitabine through PRKCE/MDR1 axis in gallbladder cancer. Cell Death Dis. 2017, 8, e2770. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.T.; Choi, R.C.; Cheung, A.W.; Zhu, J.T.; Li, J.; Chu, G.K.; Duan, R.; Cheung, J.K.; Jiang, Z.Y.; Dong, X.B.; et al. Danggui buxue tang--a Chinese herbal decoction activates the phosphorylations of extracellular signal-regulated kinase and estrogen receptor alpha in cultured MCF-7 cells. Febs Lett. 2007, 581, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.C.; Yang, K.Z.; Sun, X.F. Efficacy of auxiliary therapy with Danggui Buxue Decoction No.1 in treating patients of non-small cell lung cancer at peri-operational stage. Chin. J. Integr. Med. 2009, 15, 184–188. [Google Scholar] [CrossRef]
- McCulloch, M.; See, C.; Shu, X.J.; Broffman, M.; Kramer, A.; Fan, W.Y.; Gao, J.; Lieb, W.; Shieh, K.; Colford, J.M., Jr. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: Meta-analysis of randomized trials. J. Clin. Oncol. 2006, 24, 419–430. [Google Scholar] [CrossRef] [PubMed]
- He, C.S.; Liu, Y.C.; Xu, Z.P.; Dai, P.C.; Chen, X.W.; Jin, D.H. Astragaloside IV Enhances Cisplatin Chemosensitivity in Non-Small Cell Lung Cancer Cells Through Inhibition of B7-H3. Cell Physiol. Biochem. 2016, 40, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Rosing, H.; Schellens, J.H.; Beijnen, J.H. Simultaneous quantification of 2′,2′-difluorodeoxycytidine and 2′,2′-difluorodeoxyuridine nucleosides and nucleotides in white blood cells using porous graphitic carbon chromatography coupled with tandem mass spectrometry. Rapid Commun. Mass Spectrom 2009, 23, 3040–3050. [Google Scholar] [CrossRef]
- Mizuno, K.; Mataki, H.; Seki, N.; Kumamoto, T.; Kamikawaji, K.; Inoue, H. MicroRNAs in non-small cell lung cancer and idiopathic pulmonary fibrosis. J. Hum. Genet. 2017, 62, 57–65. [Google Scholar] [CrossRef]
- Bergman, A.M.; Pinedo, H.M.; Talianidis, I.; Veerman, G.; Loves, W.J.; van der Wilt, C.L.; Peters, G.J. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br. J. Cancer 2003, 88, 1963–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, A.M.; Pinedo, H.M.; Veerman, G.; Kuiper, C.M.; Peters, G.J. Increased sensitivity to gemcitabine of P-gP and MRP overexpressing human non-small cell lung cancer cell lines. Adv. Exp. Med. Biol. 1998, 431, 591–594. [Google Scholar]
- Lu, X. Impact of IL-12 in Cancer. Curr. Cancer Drug Targets 2017, 17, 682–697. [Google Scholar] [CrossRef]
- Tian, H.; Shi, G.; Yang, G.; Zhang, J.; Li, Y.; Du, T.; Wang, J.; Xu, F.; Cheng, L.; Zhang, X.; et al. Cellular immunotherapy using irradiated lung cancer cell vaccine co-expressing GM-CSF and IL-18 can induce significant antitumor effects. BMC Cancer 2014, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Fabianowska-Majewska, K.; Mantincic, D.; Callet Bauchu, E.; Tigaud, I.; Gandhi, V.; Lepoivre, M.; Peters, G.J.; Rolland, M.O.; Wyczechowska, D.; et al. Common resistance mechanisms to deoxynucleoside analogues in variants of the human erythroleukaemic line K562. Br. J. Haematol. 1999, 106, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.M.; Talbot, D.C.; Aherne, W.G.; Fisher, T.C.; Serafinowski, P.; Harrap, K.R. 2′-Deoxycytidine kinase deficiency is a major determinant of 2-chloro-2′-deoxyadenosine resistance in lymphoid cell lines. Clin. Cancer Res. 1995, 1, 391–398. [Google Scholar]
- Yang, S.; Li, X.; Hu, F.; Li, Y.; Yang, Y.; Yan, J.; Kuang, C.; Yang, Q. Discovery of tryptanthrin derivatives as potent inhibitors of indoleamine 2,3-dioxygenase with therapeutic activity in Lewis lung cancer (LLC) tumor-bearing mice. J. Med. Chem. 2013, 56, 8321–8331. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; von Eschenbach, A.C.; Giavazzi, R.; Fidler, I.J. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986, 46, 4109–4115. [Google Scholar] [PubMed]
- Xiang, Q.F.; Zhang, D.M.; Wang, J.N.; Zhang, H.W.; Zheng, Z.Y.; Yu, D.C.; Li, Y.J.; Xu, J.; Chen, Y.J.; Shang, C.Z. Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int. 2015, 35, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the DBD are available from the authors. |







| Parameters | dFdC | dFdU | ||
|---|---|---|---|---|
| GEM | GEM + DBD | GEM | GEM + DBD | |
| Cmax (μg/mL) | 11.60 ± 2.66 | 13.61 ± 1.64 | 0.34 ± 0.07 | 0.31 ± 0.05 |
| Tmax (h) | 0.32 ± 0.29 | 0.11 ± 0.14 | 4.00 ± 0 | 4.00 ± 0 |
| t1/2 (h) | 3.61 ± 0.59 | 3.38 ± 0.28 | 17.73 ± 3.85 | 16.89 ± 4.66 |
| AUC0–t (μg·min/mL) | 2868 ± 835 | 2814 ± 387 | 300.2 ± 47.2 | 258.5 ± 77.9 |
| AUC0–∞ (μg·min/mL) | 2914 ± 866 | 2850 ± 397 | 526.7 ± 66.1 | 491.3 ± 50.1 |
| Parameters | dFdCTP | |
|---|---|---|
| GEM | GEM + DBD | |
| Cmax (ng/mg protein) | 2.05 ± 0.43 | 3.80 ± 0.79 *** |
| Tmax (h) | 2.40 ± 0.60 | 1.09 ± 0.39 *** |
| t1/2 (h) | 7.57 ± 2.24 | 6.96 ± 2.89 |
| AUC0–t (ng·min/mg protein) | 798 ± 251 | 1266 ± 145 *** |
| AUC0–∞ (ng·min/mg protein) | 998 ± 244 | 1415 ± 176 *** |
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Product Length (bp) |
|---|---|---|---|
| Mdr1 | GTGGGGGACAGAAACAGAGA | GAACGGTAGACAAGCGATGAG | 183 |
| dCK | GGACTCTGAAAACCAGCTTTGATT | CCAGGCTTTCGTGTTTGTCTTTA | 93 |
| GAPDH-mouse | CAAGGCTGTGGGCAAGGTCA | AGGTGGAAGAGTGGGAGTTGCTG | 242 |
| GAPDH-human | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC | 101 |
| hENT1 | TCTCCAACTCTCAGCCCACCAA | CCTGCGATGCTGGACTTGACCT | 151 |
| hCNT1 | CATTACTGATCCGGCCCTACTT | TGGCGTAACCTCCGGTCAT | 75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Xu, X.; Chen, Y.; Guan, R.; Cheng, T.; Wang, Y.; Jin, R.; Song, M.; Hang, T. Danggui Buxue Decoction Sensitizes the Response of Non-Small-Cell Lung Cancer to Gemcitabine via Regulating Deoxycytidine Kinase and P-glycoprotein. Molecules 2019, 24, 2011. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24102011
Sun X, Xu X, Chen Y, Guan R, Cheng T, Wang Y, Jin R, Song M, Hang T. Danggui Buxue Decoction Sensitizes the Response of Non-Small-Cell Lung Cancer to Gemcitabine via Regulating Deoxycytidine Kinase and P-glycoprotein. Molecules. 2019; 24(10):2011. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24102011
Chicago/Turabian StyleSun, Xiyang, Xin Xu, Yanfei Chen, Rong Guan, Tingting Cheng, Ye Wang, Rui Jin, Min Song, and Taijun Hang. 2019. "Danggui Buxue Decoction Sensitizes the Response of Non-Small-Cell Lung Cancer to Gemcitabine via Regulating Deoxycytidine Kinase and P-glycoprotein" Molecules 24, no. 10: 2011. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24102011