Anti-Edematogenic and Anti-Granuloma Activity of a Synthetic Curcuminoid Analog, 5-(3,4-Dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one, in Mouse Models of Inflammation
Abstract
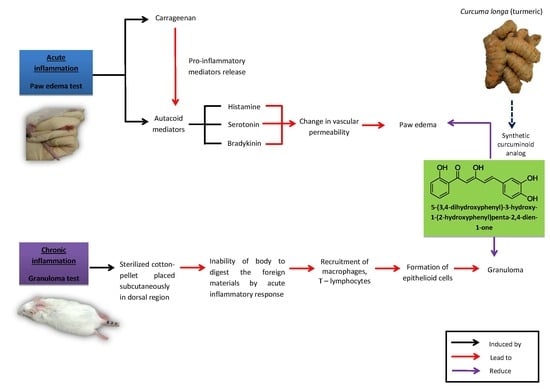
:1. Introduction
2. Results
2.1. Acute Toxicity Study
2.2. Anti-Inflammatory Study
2.2.1. Carrageenan-Induced Paw Edema Test
2.2.2. Cotton Pellet-Induced Granuloma Test
2.3. Involvement of the Histaminergic, Serotonergic and Bradykininergic System
2.3.1. Histamine-Induced Paw Edema Test
2.3.2. Serotonin-Induced Paw Edema Test
2.3.3. Bradykinin-Induced Paw Edema Test
3. Discussion
4. Materials and Methods
4.1. Synthesis, Structural Identification and Confirmation of 5-(3,4–Dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1 One
4.2. Experimental Animals, Drugs, and Chemicals
4.3. Dose Determination and Route of Administration
4.4. Acute Toxicity Study
4.5. Anti-Inflammatory Study
4.5.1. Carrageenan-Induced Paw Edema Test
4.5.2. Cotton Pellet-Induced Granuloma Test
4.6. Involvement of Histaminergic, Serotonergic and Bradykininergic Systems
4.6.1. Histamine-Induced Paw Edema Test
4.6.2. Serotonin-Induced Paw Edema Test
4.6.3. Bradykinin-Induced Paw Edema Test
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russell, R.I. Non-steroidal anti-inflammatory drugs and gastrointestinal damage—Problems and solutions. Postgrad. Med. J. 2001, 77, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Wahl, M.A. Pharmacology of curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Ali, J.; Ansari, M.J.; Raheman, Z. Curcumin: A natural antiinflammatory agent. Indian J. Pharmacol. 2005, 37, 141–147. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (congeners) made by man and mother nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Vyas, A.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013, 19, 2047–2069. [Google Scholar]
- Nandal, S.; Dhir, A.; Sharma, S.; Chopra, K. Curcumin potentiates the anti-inflammatory activity of cyclooxygenase inhibitors in the cotton pellet granuloma pouch model. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 89–93. [Google Scholar] [CrossRef]
- Agrawal, D.K.; Mishra, P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar] [CrossRef]
- Ming-Tatt, L.; Khalivulla, S.I.; Akhtar, M.N.; Mohamad, A.S.; Perimal, E.K.; Khalid, M.H.; Akira, A.; Lajis, N.; Israf, D.A.; Sulaiman, M.R. Antinociceptive activity of a synthetic curcuminoid analogue, 2,6-bis-(4-hydroxy-3-methoxybenzylidene)cyclohexanone, on nociception-induced models in mice. Basic Clin. Pharmacol. Toxicol. 2012, 110, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.W.; Faudzi, S.M.M.; Abas, F.; Aluwi, M.F.F.M.; Rullah, K.; Wai, L.K.; Bahari, M.N.A.; Ahmad, S.; Tham, C.L.; Shaari, K.; et al. Synthesis and sar study of diarylpentanoid analogues as new anti-inflammatory agents. Molecules 2014, 19, 16058–16081. [Google Scholar] [CrossRef] [PubMed]
- Eaton, F.M. Toxicity and accumulation of chloride and sulfate salts in plants. J. Agric. Res. 1942, 64, 357–399. [Google Scholar]
- Squadrito, G.L.; Postlethwait, E.M. Elucidating mechanisms of chlorine toxicity: Reaction kinetics, thermodynamics, and physiological implications. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.; Hisamuddin, N.; Ong, H.M.; Azmi, A.F.A.; Leong, S.W.; Abas, F.; Sulaiman, M.R.; Mossadeq, W.M.S. Analgesic effect of 5-(3,4-dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one in experimental animal models of nociception. Molecules 2018, 23, 2099. [Google Scholar] [CrossRef] [PubMed]
- Wilches, I.; Tobar, V.; Peñaherrera, E.; Cuzco, N.; Jerves, L.; Heyden, Y.V.; León-Tamariz, F.; Vila, E. Evaluation of anti-inflammatory activity of the methanolic extract from Jungia rugosa leaves in rodents. J. Ethnopharmacol. 2015, 173, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukarram, S.M.; Shah, A. Possible anti-inflammatory mechanism of ethyl acetate extracts of Teucrium stocksianum bioss. BMC Complement. Altern. Med. 2015, 15, 299. [Google Scholar] [CrossRef]
- Marroquin-Segura, R.; Flores-Pimentel, M.; Carreón-Sánchez, R.; Garcia-Burciaga, M.M.; Mora-Guevara, J.L.A.; Aguilar-Contreras, A.; Hernandez-Abad, V.J. The effect of the aqueous extract of Helietta parvifolia A. Gray (Rutaceae) stem bark on carrageenan-induced paw oedema and granuloma tissue formation in mice. J. Ethnopharmacol. 2009, 124, 639–641. [Google Scholar] [CrossRef]
- Toriyabe, M.; Omote, K.; Kawamata, T.; Namiki, A. Contribution of interaction between nitric oxide and cyclooxygenases to the production of prostaglandins in carrageenan-induced inflammation. Anesthesiology 2004, 101, 983–990. [Google Scholar] [CrossRef]
- Criado, P.R.; Fachini, R.; Criado, J.; Maruta, C.W.; D’apparecida, C.; Filho, M. Histamine, histamine receptors and antihistamines: New concepts. Braz. Ann. Dermatol. 2010, 85, 195–210. [Google Scholar] [CrossRef]
- Paterson, K.J.; Zambreanu, L.; Bennett, D.L.H.; McMahon, S.B. Characterisation and mechanisms of bradykinin-evoked pain in man using iontophoresis. Pain 2013, 154, 782–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.N.; Al-Sherif, G.J. Pharmacologic targets and prototype therapeutics in the kallikrein-kinin system: Bradykinin receptor agonists or antagonists. Sci. World J. 2006, 6, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Mohsin, S.S. The role of chemical mediators in the pathogenesis of inflammation with emphasis on the kinin system. Exp. Pathol. 1990, 38, 73–96. [Google Scholar] [CrossRef]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid Res. 2009, 50, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Borowski, T.; Broclawik, E. Catalytic reaction mechanism of lipoxygenase. A density functional theory study. J. Phys. Chem. B 2003, 107, 4639–4646. [Google Scholar] [CrossRef]
- Suardíaz, R.; Masgrau, L.; Lluch, J.M.; González-Lafont, A. On the regio- and stereospecificity of arachidonic acid peroxidation catalyzed by mammalian 15-lypoxygenases: A combined molecular dynamics and QM/MM study. ChemPhysChem 2013, 14, 3777–3787. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Vostinaru, O. Adverse effects and drug interactions of the non-steroidal anti-inflammatory drugs. In Non-Steroidal Anti-Inflammatory Drugs; Gamal, A.A., Ed.; InTech Open: Rijeka, Croatia, 2017; pp. 17–31. [Google Scholar]
- Osafo, N.; Agyare, C.; Obiri, D.D.; Antwi, A.O. Mechanism of action of nonsteroidal anti-inflammatory drugs. In Non-Steroidal Anti-Inflammatory Drugs; Gamal, A.A., Ed.; InTech Open: Rijeka, Croatia, 2017; pp. 2–15. [Google Scholar]
- Park, J.; Conteas, C.N. Anti-carcinogenic properties of curcumin on colorectal cancer. World J. Gastrointest. Oncol. 2010, 2, 169–176. [Google Scholar] [CrossRef]
- Rao, C.V.; Rivenson, A.; Simi, B.; Reddy, B.S. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995, 55, 259–266. [Google Scholar]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef]
- Rao, C.V.; Janakiram, N.B.; Mohammed, A. Lipoxygenase and cyclooxygenase pathways and colorectal cancer prevention. Curr. Colorectal Cancer Rep. 2013, 8, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Boland, C.R.; Chauhan, D.P. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001, 172, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Cole, H.W.; Brown, C.E.; Magee, D.E.; Magee, C.; Roudebush, R.E.; Bryant, H.U. Serotonin-induced paw edema in the rat: Pharmacological profile. Gen. Pharmacol. 1995, 26, 431–436. [Google Scholar] [CrossRef]
- Tamaddonfard, E.; Tajik, H.; Hamzeh-Gooshi, N. Effects of curcumin, morphine and naloxone on the experimentally-induced paw edema in rats. Int. J. Vet. Res. 2009, 3, 25–30. [Google Scholar]
- Brouet, I.; Ohshima, H. Curcumin, an anti-tumor promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys. Res. Commun. 1995, 206, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, N.; Basu, N. Sodium curcuminate as an anti-inflammatory agent. Indian J. Exp. Biol. 1972, 10, 235–236. [Google Scholar] [PubMed]
- Mukhopadhay, A.; Basu, N.; Ghatak, N.; Gujral, P.K. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions 1982, 12, 508–515. [Google Scholar] [CrossRef]
- Rao, T.S.; Basu, N.; Siddiqui, H.H. Anti-inflammatory activity of curcumin analogues. Indian J. Med. Res. 1982, 75, 574–578. [Google Scholar] [PubMed]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of difffuloyl methane (curcumin). A non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A. On the antioxidant mechanism of curcumin: Classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Buadonpri, W.; Wichitnithad, W.; Rojsitthisak, P.; Towiwat, P. Synthetic curcumin inhibits carrageenan-induced paw edema in rats. J. Health Res. 2009, 23, 11–16. [Google Scholar]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [PubMed]
- Liang, G.; Li, X.; Chen, L.; Yang, S.; Wu, X.; Studer, E.; Gurley, E.; Hylemon, P.B.; Ye, F.; Li, Y.; et al. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorg. Med. Chem. Lett. 2008, 18, 1525–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, H.S. Inflammation. In Rubin’s Pathology: Clinicopathologic Foundations of Medicine, 5th ed.; Rubin, R., Strayer, D.S., Rubin, E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 37–70. [Google Scholar]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Nagabushan, M.; Amonkar, A.J.; Bhide, S.V. In vitro antimutagenicity of curcumin against environmental mutagens. Food Chem. Toxicol. 1987, 25, 545–547. [Google Scholar] [CrossRef]
- Nagabushan, M.; Bhide, S.V. Nonmutagenicity of curcumin and its antimutagenic action versus chili and capsaicin. Nutr. Cancer 1986, 8, 201–210. [Google Scholar] [CrossRef]
- Šmerák, P.; Polívková, Z.; Šestáková, H.; Štětina, R.; Bárta, I.; Langová, M.; Turek, B.; Bártová, J. Antimutagenic effect of curcumin and its effect on the immune response in mice. Czech J. Food Sci. 2006, 24, 72–83. [Google Scholar] [CrossRef]
- Ragunathan, I.; Panneerselvam, N. Antimutagenic potential of curcumin on chromosomal aberrations in Allium cepa. J. Zhejiang Univ. Sci. B 2007, 8, 470–475. [Google Scholar] [CrossRef]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Shishu; Singla, A.K.; Kaur, I.P. Inhibitory effect of curcumin and its natural analogues on genotoxicity of heterocyclic amines from cooked food. Indian J. Exp. Biol. 2002, 40, 1365–1372. [Google Scholar]
- Ligumsky, M.; Golanska, E.M.; Hansen, D.G.; Kauffman, G.L. Aspirin can inhibit gastric mucosal without causing lesions in rat. Gastroenterology 1983, 84, 756–761. [Google Scholar] [CrossRef]
- Mohamad, A.S.; Akhtar, M.N.; Zakaria, Z.A.; Perimal, E.K.; Khalid, S.; Mohd, P.A.; Khalid, M.H.; Israf, D.A.; Lajis, N.H.; Sulaiman, M.R. Antinociceptive activity of a synthetic chalcone, flavokawin B on chemical and thermal models of nociception in mice. Eur. J. Pharmacol. 2010, 647, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.R.; EPerimal, K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Tasrip, N.A.; Mokhtar, F.; Zakaria, Z.A.; Lajis, N.H.; Israf, D.A. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia 2010, 81, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U.S. The anti-inflammatory activity of garden egg (Solanum aethiopicum) on egg albumin-induced oedema and granuloma tissue formation in rats. Asian Pac. J. Trop. Med. 2012, 5, 62–66. [Google Scholar] [CrossRef]
- Busnardo, T.C.P.M.; Padoani, C.; Mora, T.C.; Biavatti, M.W.; Fröde, T.S.; Bürger, C.; Claudino, V.D.; Dalmarco, E.M.; de Souzaa, M.M. Anti-inflammatory evaluation of Coronopus didymus in the pleurisy and paw oedema models in mice. J. Ethnopharmacol. 2010, 128, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. In Methods in Molecular Biology: Inflammation Protocols; Winyard, P.G., Willoughby, D.A., Eds.; Humana Press: Totowa, NJ, USA, 2003; Volume 225, pp. 115–121. [Google Scholar]
Sample Availability: Sample of the compound, DHHPD 5-(3,4-dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one, are available from F.A., S.W.L. and M.R.S. |




| Paw Thickness in mm (% Inhibition) | |||||||
|---|---|---|---|---|---|---|---|
| Group | Dose (mg/kg) | Time Interval (h) | |||||
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Vehicle | - | 2.60 ± 0.07 | 3.55 ± 0.10 d | 3.67 ± 0.09 d | 3.77 ± 0.07 d | 3.80 ± 0.07 d | 3.90 ± 0.03 d |
| DHHPD | 0.1 | 2.60 ± 0.07 | 3.44 ± 0.10 d (11.58%) | 3.44 ± 0.04 d (21.50%) a | 3.35 ± 0.03 d (35.90%) b | 3.15 ± 0.07 d (54.16%) b | 3.05 ± 0.05 d (65.38%) b |
| 0.3 | 2.59 ± 0.05 | 3.35 ± 0.02 d (20.00%) a | 3.26 ± 0.04 d (37.38%) b | 3.21 ± 0.02 d (47.00%) b | 3.06 ± 0.05 d (60.83%) b | 3.02 ± 0.04 d (66.92%) b | |
| 1 | 2.61 ± 0.04 | 3.32 ± 0.05 d (25.26%) a | 3.24 ± 0.03 d (41.12%) b | 3.14 ± 0.05 d (54.70%) b | 3.12 ± 0.03 d (57.50%) b | 2.95 ± 0.05 d (73.85%) b | |
| 3 | 2.60 ± 0.03 | 3.20 ± 0.02 d (36.84%) b | 3.05 ± 0.01 d (57.94%) b | 2.96 ± 0.03 d (69.23%) b | 2.86 ± 0.06 c (78.33%) b | 2.77 ± 0.02 (86.92%) b | |
| ASA | 100 | 2.58 ± 0.05 | 3.37 ± 0.08 d (16.84%) | 3.27 ± 0.02 d (35.51%) b | 3.16 ± 0.07 d (50.43%) b | 3.05 ± 0.02 d (60.83%) b | 2.98 ± 0.03 d (69.23%) b |
| Group | Dose (mg/kg) | Granuloma Dry Weight (mg) | Inhibition (%) |
|---|---|---|---|
| Vehicle | - | 66.84 ± 2.87 | - |
| DHHPD | 0.1 | 52.08 ± 1.82 **** | 22.08 |
| 0.3 | 45.07 ± 1.85 **** | 32.57 | |
| 1 | 41.97 ± 1.45 **** | 37.20 | |
| 3 | 33.92 ± 1.19 **** | 49.25 | |
| ASA | 100 | 33.62 ± 1.50 **** | 49.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hisamuddin, N.; Shaik Mossadeq, W.M.; Sulaiman, M.R.; Abas, F.; Leong, S.W.; Kamarudin, N.; Ong, H.M.; Ahmad Azmi, A.F.; Ayumi, R.R.; Talib, M. Anti-Edematogenic and Anti-Granuloma Activity of a Synthetic Curcuminoid Analog, 5-(3,4-Dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one, in Mouse Models of Inflammation. Molecules 2019, 24, 2614. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24142614
Hisamuddin N, Shaik Mossadeq WM, Sulaiman MR, Abas F, Leong SW, Kamarudin N, Ong HM, Ahmad Azmi AF, Ayumi RR, Talib M. Anti-Edematogenic and Anti-Granuloma Activity of a Synthetic Curcuminoid Analog, 5-(3,4-Dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one, in Mouse Models of Inflammation. Molecules. 2019; 24(14):2614. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24142614
Chicago/Turabian StyleHisamuddin, Nadia, Wan Mastura Shaik Mossadeq, Mohd Roslan Sulaiman, Faridah Abas, Sze Wei Leong, Nadhirah Kamarudin, Hui Ming Ong, Ahmad Farhan Ahmad Azmi, Rasyidah Ryta Ayumi, and Madihah Talib. 2019. "Anti-Edematogenic and Anti-Granuloma Activity of a Synthetic Curcuminoid Analog, 5-(3,4-Dihydroxyphenyl)-3-hydroxy-1-(2-hydroxyphenyl)penta-2,4-dien-1-one, in Mouse Models of Inflammation" Molecules 24, no. 14: 2614. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24142614