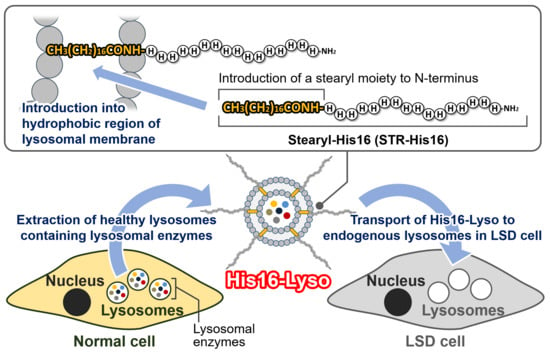
Development of Organelle Replacement Therapy Using a Stearyl-Polyhistidine Peptide against Lysosomal Storage Disease Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Lysosomes Showing Red Fluorescence
2.2. Cellular Uptake and Intracellular Distribution of His16-Lyso in HT1080 Cells
2.3. Cellular Uptake Pathway for His16-Lyso in HT1080 Cells
2.4. Cellular Uptake and Intracellular Distribution of His16-Lyso in FD Patient Fibroblasts
2.5. ERT Efficacy of His16-Lyso in FD Patient Fibroblasts
3. Materials and Methods
3.1. Peptide Synthesis and Purification
3.2. Cell Culture
3.3. Preparation of HT1080 Cells Stably Expressing LAMP-1-RFP
3.4. Isolation of Lysosomes
3.5. Preparation of His16-Lyso
3.6. Cellular Uptake of His16-Lyso
3.6.1. Flow Cytometric Analysis
3.6.2. Fluorescence Microplate Reader Analysis
3.7. CLSM Analysis
3.8. Cell Proliferation Assay (WST Assay)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Defaus, S.; Andreu, D. 1988–2018: Thirty years of drug smuggling at the nano scale. Challenges and opportunities of cell-penetrating peptides in biomedical research. Arch. Biochem. Biophys. 2019, 661, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Tokuda, Y.; Kotake, A.; Okada, H.; Takeda, S.; Kawano, T.; Nakayama, Y. Cellular uptake and in vivo distribution of polyhistidine peptides. J. Control. Release 2015, 210, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Shinagawa, M.; Kawano, T.; Iwasaki, T. Drug delivery using polyhistidine peptide-modified liposomes that target endogenous lysosome. Biochem. Biophys. Res. Commun. 2018, 501, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Lysosomal Enzymes Research Area: R&D Systems. Available online: https://www.rndsystems.com/research-area/lysosomal-enzymes (accessed on 14 May 2019).
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; Boland, B.; van der Spoel, A.C. The cell biology of disease: Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012, 199, 723–734. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lu, J.; Yue, Z. Autophagy in ageing and ageing-associated diseases. Acta Pharmacol. Sin. 2013, 34, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.T.R.; Iwanochko, R.M. Enzyme replacement therapy of Fabry disease. Mol. Neurobiol. 2005, 32, 43–50. [Google Scholar] [CrossRef]
- Bruni, S.; Loschi, L.; Incerti, C.; Gabrielli, O.; Coppa, G. V Update on treatment of lysosomal storage diseases. Acta Myol. 2007, 26, 87–92. [Google Scholar]
- Coutinho, M.F.; Prata, M.J.; Alves, S. Mannose-6-phosphate pathway: A review on its role in lysosomal function and dysfunction. Mol. Genet. Metab. 2012, 105, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Enzyme Replacement Therapy. Available online: https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/enzyme-replacement-therapy.pdf (accessed on 14 May 2019).
- Intravenous Enzyme Replacement Therapy (ERT) for Gaucher Disease. Available online: https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/intravenous-enzyme-replacement-therapy-gaucher-disease.pdf (accessed on 14 May 2019).
- Lalonde, M.-E.; Durocher, Y. Therapeutic glycoprotein production in mammalian cells. J. Biotechnol. 2017, 251, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Espejo-Mojica, Á.J.; Alméciga-Díaz, C.J.; Rodríguez, A.; Mosquera, Á.; Díaz, D.; Beltrán, L.; Díaz, S.; Pimentel, N.; Moreno, J.; Sánchez, J.; et al. Human recombinant lysosomal enzymes produced in microorganisms. Mol. Genet. Metab. 2015, 116, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Muenzer, J.; Wraith, J.E.; Beck, M.; Giugliani, R.; Harmatz, P.; Eng, C.M.; Vellodi, A.; Martin, R.; Ramaswami, U.; Gucsavas-Calikoglu, M.; et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet. Med. 2006, 8, 465–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhoen, S.; Laufer, S.; Restle, T. Recent developments in peptide-based nucleic acid delivery. Int. J. Mol. Sci. 2008, 9, 1276–1320. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Akita, H.; Kogure, K.; Gräslund, A.; Langel, Ü.; Harashima, H.; Futaki, S. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc. Chem. Res. 2012, 45, 1132–1139. [Google Scholar] [CrossRef]
- Eskelinen, E.-L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Aspects Med. 2006, 27, 495–502. [Google Scholar] [CrossRef]
- Andrejewski, N.; Punnonen, E.L.; Guhde, G.; Tanaka, Y.; Lüllmann-Rauch, R.; Hartmann, D.; von Figura, K.; Saftig, P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J. Biol. Chem. 1999, 274, 12692–12701. [Google Scholar] [CrossRef]
- Richard, J.P.; Melikov, K.; Vives, E.; Ramos, C.; Verbeure, B.; Gait, M.J.; Chernomordik, L.V.; Lebleu, B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003, 278, 585–590. [Google Scholar] [CrossRef]
- Tréhin, R.; Merkle, H.P. Chances and pitfalls of cell penetrating peptides for cellular drug delivery. Eur. J. Pharm. Biopharm. 2004, 58, 209–223. [Google Scholar] [CrossRef]
- Cascales, L.; Henriques, S.T.; Kerr, M.C.; Huang, Y.-H.; Sweet, M.J.; Daly, N.L.; Craik, D.J. Identification and characterization of a new family of cell-penetrating peptides: Cyclic cell-penetrating peptides. J. Biol. Chem. 2011, 286, 36932–36943. [Google Scholar] [CrossRef]
- Kosuge, M.; Takeuchi, T.; Nakase, I.; Jones, A.T.; Futaki, S. Cellular internalization and distribution of arginine-rich peptides as a function of extracellular peptide concentration, serum, and plasma membrane associated proteoglycans. Bioconjug. Chem. 2008, 19, 656–664. [Google Scholar] [CrossRef]
- Youngblood, D.S.; Hatlevig, S.A.; Hassinger, J.N.; Iversen, P.L.; Moulton, H.M. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconjug. Chem. 2007, 18, 50–60. [Google Scholar] [CrossRef]
- Shu, L.; Vivekanandan-Giri, A.; Pennathur, S.; Smid, B.E.; Aerts, J.M.F.G.; Hollak, C.E.M.; Shayman, J.A. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int. 2014, 86, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, A.; Sato, A.; Kawashima, M.; Nitta, K.; Yumura, W.; Sugino, N.; Nihei, H.; Natori, Y. A simple procedure for the isolation of rat kidney lysosomes. Kidney Int. 1998, 54, 275–278. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, T.; Okamoto, R.; Kawano, T.; Iwasaki, T. Development of Organelle Replacement Therapy Using a Stearyl-Polyhistidine Peptide against Lysosomal Storage Disease Cells. Molecules 2019, 24, 2995. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24162995
Hayashi T, Okamoto R, Kawano T, Iwasaki T. Development of Organelle Replacement Therapy Using a Stearyl-Polyhistidine Peptide against Lysosomal Storage Disease Cells. Molecules. 2019; 24(16):2995. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24162995
Chicago/Turabian StyleHayashi, Taiki, Riku Okamoto, Tsuyoshi Kawano, and Takashi Iwasaki. 2019. "Development of Organelle Replacement Therapy Using a Stearyl-Polyhistidine Peptide against Lysosomal Storage Disease Cells" Molecules 24, no. 16: 2995. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24162995