Transcriptome Analysis Identifies Key Genes Responsible for Red Coleoptiles in Triticum Monococcum
Abstract
:1. Introduction
2. Results
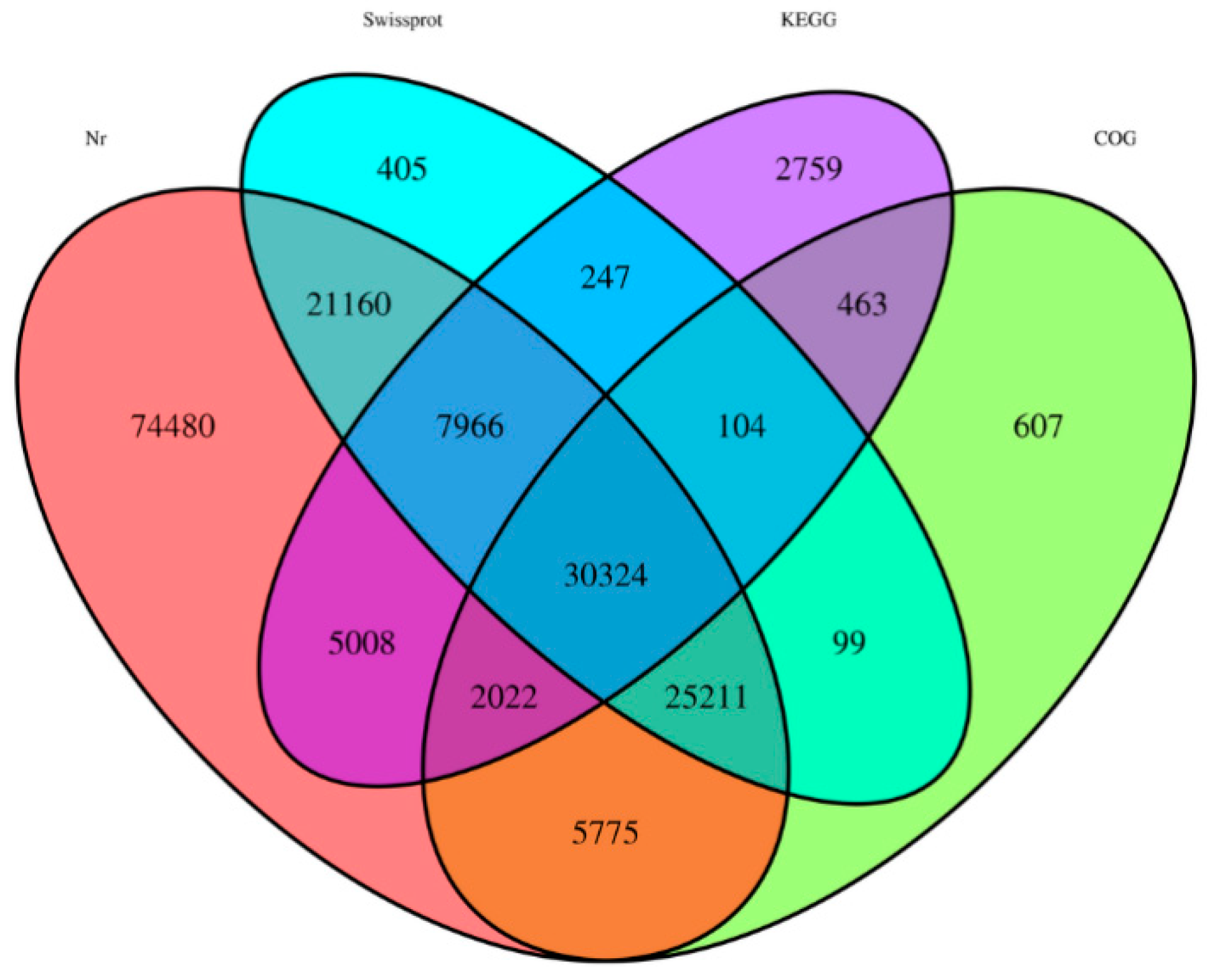
2.1. High-Throughput RNA Sequencing
2.2. Expression Profiles of Structural Genes Associated with Anthocyanin Biosynthesis
2.3. Sequence Characteristics and Expression Profiles of Regulatory Genes Associated with Anthocyanin Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of Total RNA and cDNA
4.3. Transcriptome Analysis
4.4. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shoeva, O.Y.; Gordeeva, E.I.; Arbuzova, V.S.; Khlestkina, E.K. Anthocyanins participate in protection of wheat seedlings from osmotic stress. Cereal Res. Commun. 2017, 45, 1–10. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Sarma, A.D.; Sharma, R. Anthocyanin-DNA copigmentation complex:mutual protection against oxidative damage. Phytochemistry 1999, 52, 1313–1318. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation ofanthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Bartłomiej, S.; Justyna, R.K.; Ewa, N. Bioactive compounds in cerealgrains—Occurrence, structure, technological significance and nutritionalbenefits—A review. Food Sci. Tech. Int. 2012, 18, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.B.; Raasch, J.A.; Kramer, L.C.; Neumann, P.; Wielgus, S.M.; Austin-Phillips, S.; Jiang, J. Sgt1, but not Rar1, is essential for the RB-mediated broad-spectrum resistance to potato late blight. BMC Plant Biol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful modelfor the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoidbiosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Styles, E.D.; Ceska, O.; Seah, K.T. Developmental differences in action of R and B alleles in Maize. Can. J. Genet. Cytal. 1973, 15, 59–72. [Google Scholar] [CrossRef]
- Atchley, W.R.; Wollenberg, K.R.; Fitch, W.M.; Terhalle, W.; Dress, A.W. Correlations among amino acid sites in bHLH protein domains: An information theoretic analysis. Mol. Biol. Evolut. 2000, 17, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liao, L.; Zhou, H.; Gu, C.; Wang, L.; Han, Y. A small indelmutation in an anthocyanin transporter causes variegated colouration ofpeach flowers. J. Exp. Bot. 2015, 66, 7227–7239. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Hou, X.J.; Zhang, B.; Chen, W.J.; Liu, D.C.; Liu, B.L.; Zhang, H.G. Identification of a candidate gene for Rc-D1, a locus controlling red coleoptile colour in wheat. Cereal Res. Commun. 2016, 44, 35–46. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, T.; Nan, W.; Jeewani, D.C.; Niu, Y.; Li, C.; Wang, Y.; Shi, X.; Wang, C.; Wang, J.; et al. Two transcription factors tappm1 and tappb1 co-regulate the anthocyanin biosynthesis in purple pericarp of wheat. J. Exp. Bot. 2018, 65, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Ye, G.J.; Zong, Y.; Zhang, B.; Chen, W.J.; Liu, B.L.; Zhang, H.G. AetMYC1, the candidate gene controlling the red coleoptile trait in Aegilops tauschii coss. accession as77. Molecules 2017, 22, 2259. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Debeaujon, I.; Jond, C.; Pelletier, G.; Caboche, M.; Lepiniec, L. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of dfr and ban genes in Arabidopsis siliques. Plant Cell 2000, 12, 1863–1878. [Google Scholar] [CrossRef] [PubMed]
- Spelt, C.; Quattrocchio, F.; Mol, J.N.; Koes, R. Anthocyanin1 of petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 2000, 12, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, S.M.; Zhang, K.P.; Chen, W.J.; Zhang, B.; Wang, D.W.; Liu, B.L.; Zhang, H.G. ThMYC4E, candidate blue aleurone 1 gene controlling the associated trait in Triticum aestivum. PLoS ONE 2017, 12, e0181116. [Google Scholar] [CrossRef] [PubMed]
- Shoeva, O.; Gordeeva, E.; Khlestkina, E. The regulation of anthocyanin synthesis in the wheat pericarp. Molecules 2014, 19, 20266–20279. [Google Scholar] [CrossRef] [PubMed]
- Borevitz, J.O.; Xia, Y.; Blount, J.; Dixon, R.A.; Lamb, C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 2000, 12, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchio, F.; Wing, J.; Woude, K.V.D.; Souer, E.; Vetten, N.D.; Mol, J.; Koes, R. Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell 1999, 11, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Ogasawara, F.; Sato, K.; Higo, H.; Minobe, Y. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol. 2007, 143, 1252–1268. [Google Scholar] [CrossRef] [PubMed]
- Peel, G.J.; Pang, Y.Z.; Modolo, L.V.; Dixon, R.A. The lap1 myb transcription factor orchestrates anthocyanidin biosynthesis and glycosylation in medicago. Plant J. 2010, 59, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007, 48, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Takos, A.M.; Jaffe, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Sun, W.; Lv, H.Y.; Luo, M.; Zeng, S.H.; Pattanaik, S.; Yuan, L.; Wang, Y. A R2R3-MYB transcription factor from epimedium sagittatum regulates the flavonoid biosynthetic pathway. PLoS ONE 2013, 8, e70778. [Google Scholar] [CrossRef] [PubMed]
- Khlestkina, E.K.; Pestsova, E.G.; Roder, M.S.; Borner, A. Molecular mapping, phenotypic expression and geographical distribution of genes determining anthocyanin pigmentation of coleoptiles in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2002, 104, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Himi, E.; Taketa, S. Isolation of candidate genes for the barley Ant1 and wheat Rc genes controlling anthocyanin pigmentation in different vegetative tissues. Mol. Genet. Genom. 2015, 290, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.J.; Wei, L.; Chen, W.J.; Zhang, B.; Liu, B.L.; Zhang, H.G. Frame-shift mutation causes the function loss of TaMYB-A1 regulating anthocyanin biosynthesis in Triticum aestivum. Cereal Res. Commun. 2017, 45, 35–46. [Google Scholar] [CrossRef]
- Cao, D.; Ye, G.J.; Zong, Y.; Zhang, B.; Chen, W.J.; Liu, B.L.; Zhang, H.G. Diversity and distribution of Rc1 alleles in Triticum urartu from the Middle East. Genet. Resour. Crop. Evolut. 2017, 35, 637–644. [Google Scholar] [CrossRef]
- Kumar, R.; Ichihashi, Y.; Kimura, S.; Chitwood, D.H.; Headland, L.R.; Peng, J.; Maloof, J.N.; Sinha, N.R. A high-throughput method for Illumina RNA-seq library preparation. Front. Plant Sci. 2012, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, S.M.; Chen, W.J.; Zhang, B.; Liu, D.C.; Liu, B.L.; Zhang, H.G. Transcriptome analysis of purple pericarps in common wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155428. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.R.; Duan, J.J.; Huo, D.; Shi, Q.Q.; Niu, L.X.; Zhang, Y.L. Transcriptomic analysis reveals transcription factors related to leaf anthocyanin biosynthesis in paeonia qiui. Molecules 2017, 22, 2186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Luo, X.; Wu, C.Y.; Cao, S.Y.; Zhou, Y.F.; Jie, B.; Cao, Y.L.; Meng, H.J.; Wu, G.L. Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in red and green walnut (juglans regia L.). Molecules 2017, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Himi, E.; Maekawa, M.; Miura, H.; Noda, K. Development of pcr markers for TaMYB10 related tor-1, red grain color gene in wheat. Theor. Appl. Genet. 2011, 122, 1561–1576. [Google Scholar] [CrossRef] [PubMed]
- Strygina, K.V.; Khlestkina, E.K. MYC gene family in cereals: Transformations during evolution of hexaploid bread wheat and its relatives. Mol. Biol. 2017, 51, 772–779. [Google Scholar] [CrossRef]
- Wang, C.; Shu, Q. Fine mapping and candidate gene analysis of purple pericarp gene Pb in rice (Oryza sativa L.). Chin. Sci. Bull. 2007, 52, 3097–3104. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, X.; Gao, J.; Guo, Y.; Huang, Z.; Du, Y. The tomatohoffman’s anthocyaninless gene encodes a bHLH transcription factor involved in anthocyanin biosynthesis that is developmentally regulated and induced by low temperatures. PLoS ONE 2016, 11, e0151067. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, F.C.; Abdalla, O.; Mujeeb-Kazi, A.; Kazi, A.G.; Xu, S.S.; Gosman, N.; Lagudah, E.S.; Bonnett, D.; Sorrells, M.E.; Tsujimoto, H. Synthetic hexaploids: Harnessing species of the primary gene pool for wheat improvement. J. Plant Breed. Rev. 2013, 37, 35–122. [Google Scholar]
- United States Department of Agriculture Agricultural Research Service. Available online: https://www.ars-grin.gov/npgs/sitelist.html (accessed on 18 September 2015).
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Romualdi, C.; Bortoluzzi, S.; D’Alessi, F.; Danieli, G.A. Ideg6: A web tool for detection of differentially expressed genes in multiple tag sampling experiments. Physiol. Genom. 2003, 12, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Kyoto Encyclopedia of Genes and Genomes. Available online: http://www.genome.jp/kegg/ (accessed on 3 December 2018).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evolut. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of DNA of all Triticum monococcum accessions are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, D.; Fan, J.; Xi, X.; Zong, Y.; Wang, D.; Zhang, H.; Liu, B. Transcriptome Analysis Identifies Key Genes Responsible for Red Coleoptiles in Triticum Monococcum. Molecules 2019, 24, 932. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050932
Cao D, Fan J, Xi X, Zong Y, Wang D, Zhang H, Liu B. Transcriptome Analysis Identifies Key Genes Responsible for Red Coleoptiles in Triticum Monococcum. Molecules. 2019; 24(5):932. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050932
Chicago/Turabian StyleCao, Dong, Jiequn Fan, Xingyuan Xi, Yuan Zong, Dongxia Wang, Huaigang Zhang, and Baolong Liu. 2019. "Transcriptome Analysis Identifies Key Genes Responsible for Red Coleoptiles in Triticum Monococcum" Molecules 24, no. 5: 932. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24050932



