Trinuclear NiII-LnIII-NiII Complexes with Schiff Base Ligands: Synthesis, Structure, and Magnetic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization
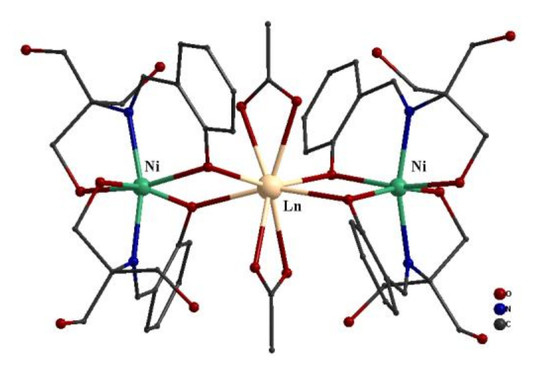
2.2. Description of the Structure
2.3. Magnetic Measurements
3. Materials and Methods
3.1. General and Spectroscopic Measurements
3.2. Compound Preparations
3.2.1. General Synthetic Route for [Ni2Ln(H3L)4(O2CMe)2](NO3) (1–4)
3.2.2. [Ni2Sm(H3L)4(O2CMe)2](NO3) (1)
3.2.3. [Ni2Eu(H3L)4(O2CMe)2](NO3) (2)
3.2.4. [Ni2Gd(H3L)4(O2CMe)2](NO3) (3)
3.2.5. [Ni2Tb(H3L)4(O2CMe)2](NO3) (4)
3.3. Single Crystal X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References and Notes
- See for example: Chakraborty, A.; Goura, J.; Bag, P.; Chandrasekhar, V. NiII-LnIII Heterometallic Complexes as Single-Molecule Magnets. Eur. J. Inorg. Chem. 2019, 1180–1200. [Google Scholar] [CrossRef]
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3d-4f Complexes as Single Molecule Magnets. Chem. Asian J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef] [PubMed]
- Single-Molecule Magnets: Molecular Architectures and Building Blocks for Spintronics; Holyńska, M. (Ed.) Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 19–28. [Google Scholar]
- Benchini, A.; Benelli, C.; Caneschi, A.; Carlin, R.L.; Dei, A.; Gatteschi, D. Crystal and Molecular Structure of and Magnetic Coupling in two Complexes Containing Gadolimium(III) and Copper(II) Ions. J. Am. Chem. Soc. 1985, 107, 8128–8136. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Dupuis, A.; Laurent, J.P. A Genuine Example of a Discrete Bimetallic (Cu, Gd) Complex: Structural Determination and Magnetic Properties. Inorg. Chem. 1996, 35, 2400–2402. [Google Scholar] [CrossRef] [PubMed]
- Pasatoiu, T.D.; Sutter, J.-P.; Madalan, A.M.; Fellah, F.Z.C.; Duhayon, C.; Andruh, M. Preparation, Crystal Structures, and Magnetic Features for a Series of Dinuclear [NiIILnIII] Schiff-Base Complexes: Evidence for Slow Relaxation of the Magnetization for the DyIII Derivative. Inorg. Chem. 2011, 50, 5890–5898. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, V.; Pandian, B.M.; Azhakar, R.; Vittal, J.J.; Clérac, R. Linear Trinuclear Mixed-Metal CoII-GdIII-CoII Single-Molecule Magnet: [L2Co2Gd][NO3].2CHCl3 (LH3 = (S)P[N(Me)N=CH-C6H3-2-OH-3-OMe]3). Inorg. Chem. 2007, 46, 5140–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekhar, V.; Pandian, B.M.; Vittal, J.J.; Clérac, R. Synthesis, Structure, and Magnetism of Heterobimetallic Trinuclear Complexes {[L2Co2Ln][X]} [Ln = Eu, X = Cl; Ln = Tb, Dy, Ho, X = NO3; LH3 = (S)P[N(Me)N=CH-C6H3-2-OH-3-OMe]3]: A 3d-4f Family of Single-Molecule Magnets. Inorg. Chem. 2009, 48, 1148–1157. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Costes, J.-P.; Kishima, Y.; Kojima, M.; Sunatsuki, Y.; Bréfuel, N.; Tuchagues, J.-P.; Vendier, L.; Wernsdorfer, W. Face-Sharing Heterotrinuclear MII-LnIII-MII (M = Mn, Fe, Co, Zn; Ln = La, Gd, Tb, Dy) Complexes: Synthesis, Structures, and Magnetic Properties. Inorg. Chem. 2010, 49, 9125–9135. [Google Scholar] [CrossRef]
- The literature listed below covers the period 2000-2019.
- Chandrasekhar, V.; Pandian, B.M.; Boomishankar, R.; Steiner, A.; Vittal, J.J.; Houri, A.; Clérac, R. Trinuclear Heterobimetallic Ni2Ln Complexes [L2Ni2Ln][ClO4] (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, and Er; LH3 = (S)P[N(Me)N=CH-C6H3-2-OH-3-OMe]3]: From Simple Paramagnetic Complexes to Single-Molecule Magnet Behavior. Inorg. Chem. 2008, 47, 4918–4929. [Google Scholar] [CrossRef]
- Singh, S.K.; Rajeshkumar, T.; Chandrasekhar, V.; Rajaraman, G. Theoretical Studies on {3d-Gd} and {3d-Gd-3d} Complexes: Effect of Metal Substitution on the Effective Exchange Interaction. Polyhedron 2013, 66, 81–86. [Google Scholar] [CrossRef]
- Costes, J.-P.; Yamaguchi, T.; Kojima, M.; Vendier, L. Experimental Evidence for the Participation of 5d GdIII Orbitals in the Magnetic Interaction in Ni-Gd Complexes. Inorg. Chem. 2009, 48, 5555–5561. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.-X.; Zhu, Z.-X.; Lu, X.-Y.; Deng, X.-W.; Jing, S. Synthetic Ability of Dinuclear Mesocates Containing 1,3-bis(diazinecarboxamide)benzene Bridging Ligands to Form Complexes of Increased Nuclearity. Crystal Structures, Magnetic Properties and Theoretical Studies. Dalton Trans. 2016, 45, 10689–10695. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Enders, M.; Groẞhauser, M.; Hiller, M.; Müller, D.; Wadepohl, H. Solution and Solid State Structures and Magnetism of a Series of Linear Trinuclear Compounds with a Hexacoordinate LnIII and two Terminal NiII Centers. Dalton Trans. 2017, 46, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-R.; Zhang, J.-L.; Liang, F.-Y.; Yang, K.; Liu, S.-J.; Liao, J.-S.; Liu, C.-M. TbIII/3d-TbIII Clusters Derived from a 1,4,7-triazacyclononane-based Hexadentate Ligand with Field-Induced Slow Magnetic Relaxation and Oxygen-Sensitive Luminescence. New J. Chem. 2019, 43, 4067–4074. [Google Scholar] [CrossRef]
- Upadhyay, A.; Komatireddy, N.; Ghirri, A.; Tuna, F.; Langley, S.K.; Srivastava, A.K.; Sañudo, E.C.; Moubaraki, B.; Murray, K.S.; McInnes, E.J.L.; et al. Synthesis and Magnetothermal Properties of a Ferromagnetically Coupled NiII-GdIII-NiII Cluster. Dalton Trans. 2014, 43, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Das, C.; Langley, S.K.; Murray, K.S.; Srivastava, A.K.; Shanmugam, M. Heteronuclear Ni(II)-Ln(III) (Ln = La, Pr, Tb, Dy) Complexes: Synthesis and Single-Molecule Magnet Behaviour. Dalton Trans. 2016, 45, 3616–3626. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, F.; Song, Y.-M.; Feng, X.-F.; Luo, M.-B.; Liao, Z.-W.; Sun, G.-M.; Tian, X.-Z.; Yuan, Z.-J. The First One-Pot Synthesis of Multinuclear 3d-4f Metal-Organic Compounds Involving a Polytopic N,O-Donor Ligand Formed in Situ. Cryst. Growth Des. 2012, 12, 2158–2161. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sunatsuki, Y.; Kojima, M.; Akashi, H.; Tsuchimoto, M.; Re, N.; Osa, S.; Matsumoto, N. Ferromagnetic NiII-GdIII Interactions in Complexes with NiGd, NiGdNi, and NiGdGdNi Cores Supported by Tripodal Ligands. Chem. Commun. 2004, 1048–1049. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sunatsuki, Y.; Ishida, H.; Kajima, M.; Akashi, H.; Re, N.; Matsumoto, N.; Pochaba, A.; Mroziński, J. Synthesis, Structures, and Magnetic Properties of Doubly Face-Sharing Heterotrinuclear NiII-LnIII-NiII (Ln = Eu, Gd, Tb, and Dy) Complexes. Bull. Chem. Soc. Jpn. 2008, 81, 598–605. [Google Scholar] [CrossRef]
- Xu, Z.; Read, P.W.; Hibbs, D.E.; Hursthouse, M.B.; Abdul Malik, K.M.; Patrick, B.O.; Rettig, S.J.; Seid, M.; Summers, D.A.; Pink, M.; et al. Coaggregation of Paramagnetic d- and f-Block Metal Ions with a Podant-Framework Amino Phenol Ligand. Inorg. Chem. 2000, 39, 508–516. [Google Scholar] [CrossRef]
- Barta, C.A.; Bayly, S.R.; Read, P.W.; Patrick, B.O.; Thompson, R.C.; Orvig, C. Molecular Architectures for Trimetallic d/f/d Complexes: Structural and Magnetic Properties of a LnNi2 Core. Inorg. Chem. 2008, 47, 2280–2293. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Forsyth, C.M.; Junk, P.C.; Leary, S.G. A Rare Earth Alloy as a Synthetic Reagent: Contrasting Homometallic Rare Earth and Heterobimetallic Outcomes. New J. Chem. 2006, 30, 592–596. [Google Scholar] [CrossRef]
- Ahmed, N.; Das, C.; Vaidya, S.; Kumar Srivastava, A.; Langley, S.K.; Murray, K.S.; Shanmugam, M. Probing the Magnetic and Magnetothermal Properties of M(II)-Ln(III) Complexes (where M(II) = Ni or Zn; Ln(III) = La or Pr or Gd). Dalton Trans. 2014, 43, 17375–17384. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, A.; Kundu, S.; Biswas, S.; Mota, A.J.; Colacio, E.; Chandrasekhar, V. Linear {NiII-LnIII-NiII} Complexes Containing Twisted Planar Ni(μ-phenolate)2Ln Fragments: Synthesis, Structure, and Magnetothermal Properties. Chem. Asian. J. 2014, 9, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Donnadieu, B.; Gheorghe, R.; Novitchi, G.; Tuchagues, J.-P.; Vendier, L. Di- or Trinuclear 3d-4f Schiff Base Complexes: The Role of Anions. Eur. J. Inorg. Chem. 2008, 5235–5244. [Google Scholar] [CrossRef]
- Shiga, T.; Ito, N.; Hidaka, A.; Okawa, H.; Kitagawa, S.; Ohba, M. Series of Trinuclear NiIILnIIINiII Complexes Derived from 2,6-Di(acetoacetyl)pyridine: Synthesis, Structure, and Magnetism. Inorg. Chem. 2007, 46, 3492–3501. [Google Scholar] [CrossRef] [PubMed]
- Bayly, S.R.; Xu, Z.; Patrick, B.O.; Rettig, S.J.; Pink, M.; Thompson, R.C.; Orvig, C. d/f Complexes with Uniform Coordination Geometry: Structural and Magnetic Properties of an LnNi2 Core Supported by a Hepradentate Amine Phenol Ligand. Inorg. Chem. 2003, 42, 1576–1583. [Google Scholar] [CrossRef]
- Mustapha, A.; Reglinski, J.; Kennedy, A.R. The Use of Hydrogenated Schiff Base Ligands in the Synthesis of Multi-Metallic Compounds. Inorg. Chim. Acta 2009, 362, 1267–1274. [Google Scholar] [CrossRef]
- Wen, H.-R.; Dong, P.-P.; Liu, S.-J.; Liao, J.-S.; Liang, F.-Y.; Liu, C.-M. 3d-4f Heterometallic Trinuclear Complexes Derived from Amine-Phenol Tripodal Ligands Exhibiting Magnetic and Luminescent Properties. Dalton Trans. 2017, 46, 1153–1162. [Google Scholar] [CrossRef]
- Sui, Y.; Hu, R.-H.; Liu, D.-S.; Wu, Q. Adjustment of the Structures and Biological Activities by the Ratio of NiL to RE for Two Sets of Schiff Base Complexes [(NiL)nRE] (n = 1 or 2; RE = La or Ce). Inorg. Chem. Comm. 2011, 14, 396–398. [Google Scholar] [CrossRef]
- Ali Güngör, S.; Kose, M. Synthesis, Crystal Structure, Photoluminescence and Electrochemical Properties of a Sandwiched Ni2Ce Complex. J. Mol. Struct. 2017, 1150, 274–278. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, A. Coordination of Metalloligand [NiL] (H2L = Salen Type N2O2 Schiff Base Ligand) to the f-Block Elements: Structural Elucidation and Spectrophotometric Investigation. Inorg. Chim. Acta 2016, 442, 64–69. [Google Scholar] [CrossRef]
- Cristovao, B.; Kłak, J.; Miroslaw, B. Synthesis, Crystal Structures and Magnetic Behavior of NiII-4f-NiII Compounds. Polyhedron 2012, 43, 47–54. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Pełka, R.; Miroslaw, B.; Hnatejko, Z. Heterometallic Trinuclear 3d-4f-3d Compounds Based on a Hexadentate Schiff Base Ligand. Polyhedron 2014, 68, 180–190. [Google Scholar] [CrossRef]
- Trieu, T.N.; Nguyen, M.H.; Abram, U.; Nguyen, H.H. Synthesis and Structures of New Trinuclear MIILnMII (M = Ni, Co; Ln = Gd, Ce) Complexes with 2,6-Bis(acetobenzoyl)pyridine. Z. Anorg. Allg. Chem. 2015, 641, 863–870. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Jegathesh, J.J.; Takiden, A.; Hauenstein, D.; Pham, C.T.; Le, C.D.; Abram, U. 2,6-Dipicolinoylbis(N,N-dialkylthioureas) as Versatile Building Blocks for Oligo- and Polynuclear Architectures. Dalton Trans. 2016, 45, 10771–10779. [Google Scholar] [CrossRef] [Green Version]
- Bhunia, A.; Yadav, M.; Lan, Y.; Powell, A.K.; Menges, F.; Riehn, C.; Niedner-Schatteburg, G.; Jana, P.P.; Riedel, R.; Harms, K.; et al. Trinulcear Nickel-Lanthanide Compounds. Dalton Trans. 2013, 42, 2445–2450. [Google Scholar] [CrossRef]
- Chesman, A.S.R.; Turner, D.R.; Moubaraki, B.; Murray, K.S.; Deacon, G.B.; Batten, S.R. Synthesis and Magnetic Properties of a Series of 3d/4f/3d Heterometallic Trinuclear Complexes Incorporating in Situ Ligand Formation. Inorg. Chim. Acta 2012, 389, 99–106. [Google Scholar] [CrossRef]
- Papatriantafyllopoulou, C.; Estrader, M.; Efthymioua, C.G.; Dermitzaki, D.; Gkotsis, K.; Terzis, A.; Diaz, C.; Perlepes, S.P. In search for mixed transition metal/lanthanide single-molecule magnets: Synthetic routes to NiII/TbIII and NiII/DyIII clusters featuring a 2-pyridyl oximate ligand. Polyhedron 2009, 28, 1652–1655. [Google Scholar] [CrossRef]
- Kalogridis, C.; Palacios, M.A.; Rodríguez-Diéguez, A.; Mota, A.J.; Choquesillo-Lazarte, D.; Brechin, E.K.; Colacio, E. Heterometallic Oximato-Bridged Linear Trinuclear NiII−MIII−NiII (MIII = Mn, Fe, Tb) Complexes Constructed with the fac-O3 [Ni(HL)3]− Metalloligand (H2L = pyrimidine-2-carboxamide oxime): A Theoretical and Experimental Magneto-Structural Study. Eur. J. Inorg. Chem. 2011, 2011, 5225–5232. [Google Scholar] [CrossRef]
- Burkovskaya, N.P.; Orlova, E.V.; Kiskin, M.A.; Efimov, N.N.; Bogomyakov, A.S.; Fedin, M.V.; Kolotilov, S.V.; Minin, V.V.; Aleksandrov, G.G.; Sidorov, A.A.; et al. Synthesis, structure, and magnetic properties of heterometallic trinuclear complexes {MII–LnIII–MII} (MII = Ni, Cu; LnIII = La, Pr, Sm, Eu, Gd). Russ. Chem. Bull Int. Ed. 2011, 60, 2490–2503. [Google Scholar] [CrossRef]
- Efthymiou, C.G.; Georgopoulou, A.N.; Papatriantafyllopoulou, C.; Terzis, A.; Raptopoulou, C.P.; Escuer, A.; Perlepes, S.P. Initial employment of di-2-pyridyl ketone as a route to nickel(ii)/lanthanide(iii)clusters: Triangular Ni2Ln complexes. Dalton Trans. 2010, 39, 8603–8605. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, A.N.; Efthymiou, C.G.; Papatriantafyllopoulou, C.; Psycharis, V.; Raptopoulou, C.P.; Manos, M.; Tasiopoulos, A.J.; Escuer, A.; Perlepes, S.P. Triangular NiII2LnIII and NiII2YIII complexes derived from di-2-pyridyl ketone: Synthesis, structures and magnetic properties. Polyhedron 2011, 30, 2978–2986. [Google Scholar] [CrossRef]
- Unit cell determination of 3 from EtOH/Et2O/n-hexane: a = 10.089(2), b = 18.200(4), c = 19.871(4) Å, V = 3648.77 Å3. See Table 3.
- Mounika, K.; Anupama, B.; Pragathi, J.; Gyanakumari, C. Synthesis, Characterization and Biological Activity of a Schiff Base Derived from 3-Ethoxy Salicylaldehyde and 2-Amino Benzoic Acid and its Transition Metal Complexes. J. Sci. Res. 2010, 2, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Deacon, G.B.; Phillips, R.J. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Patil, K.C.; Chandrashekhar, G.V.; George, M.V.; Rao, C.N.R. Infrared Spectra and Thermal Decompositions of Metal Acetates and Dicarboxylates. Can. J. Chem. 1968, 46, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Ribot, F.; Toledano, P.; Sanchez, C. X-ray and Spectroscopic Investigations of the Structure of Yttrium Acetate Tetrahydrate. Inorg. Chim. Acta 1991, 185, 239–245. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Llunell, M.; Casanova, D.; Girera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.0; Universitat de Barcelona: Barselona, Spain, 2010. [Google Scholar]
- Batten, S.R.; Robson, R. Interpenetrating Nets: Ordered, Periodic Entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Blatov, V.A.; Carlucci, L.; Ciani, G.; Proseprio, D.M. Interpenetrating metal–organic and inorganic 3D networks: A computer-aided systematic investigation. Part I. Analysis of the Cambridge structural database. CrystEngComm 2004, 6, 377–395. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Savvidou, A.; Raptopoulou, C.P.; Psycharis, V. 3D supramolecular networks based on hydroxyl-rich Schiff-base copper(II) complexes. Polyhedron 2018, 152, 125–137. [Google Scholar] [CrossRef]
- Miller, S.J. Interpenetrating Lattices Materials of the Future. Adv. Mater. 2001, 13, 525–527. [Google Scholar] [CrossRef]
- Raptopoulou, C.P.; Sanakis, Y.; Psycharis, V.; Pissas, M. Zig-zag [MnIII4] Clusters from Polydentate Schiff Base Ligands. Polyhedron 2013, 64, 181–188. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynuclear d- and f-Block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Rigaku/MSC. Crystal Clear; Rigaku/MSC Inc.: The Woodlands, TX, USA, 2005. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- DIAMOND—Crystal and Molecular Structure Visualization; Ver. 3.1, Crystal Impact; K. Brandenburg & H. Putz GbR: Bonn, Germany, 2008.







| Tb1—O1 | 2.323 (4) | Ni—N | 2.038 (6) |
| Tb1—O1′ | 2.323 (4) | Ni—N‴ | 2.038 (6) |
| Tb1—O1″ | 2.323 (4) | Ni—O1‴ | 2.074 (5) |
| Tb1—O1‴ | 2.323 (4) | Ni—O1 | 2.074 (5) |
| Tb1—O5 | 2.469 (4) | Ni—O3 | 2.085 (6) |
| Tb1—O5″ | 2.469 (4) | Ni—O3‴ | 2.085 (6) |
| Tb1—O5′ | 2.469 (4) | Tb1—O5‴ | 2.469 (4) |
| Interaction | D···A (Å) | H···A (Å) | D-H···A (°) | Symmetry Operation |
|---|---|---|---|---|
| O(2)-H(2O)···O(5) | 2.711 | 1.884 | 172.6 | x, y, z |
| O(3)-H(3O)···O(1E) | 2.611 | 1.873 | 152.8 | 0.5 + x, −y, 0.5 − z |
| O(4)-H(4O)···O(2) | 2.748 | 1.914 | 171.7 | 2 − x, −y, 1 − z |
| 4·4EtOH·4H2O | |
|---|---|
| Formula | C56H94N5Ni2O31Tb |
| Fw | 1609.70 |
| Space group (system) | Pnnn (orthorhombic) |
| a (Å) | 10.0634(2) |
| b (Å) | 18.2467(3) |
| c (Å) | 19.8388(4) |
| V (Å3) | 3642.88(12) |
| Z | 2 |
| T (oC) | −113 |
| Radiation | Cu Kα 1.54178 |
| ρcalcd, g cm−3 | 1.468 |
| μ, mm−1 | 6.017 |
| Reflections with I > 2σ(I) | 2053 |
| R1a | 0.0781 |
| wR2 a | 0.2045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgopoulou, A.N.; Pissas, M.; Psycharis, V.; Sanakis, Y.; Raptopoulou, C.P. Trinuclear NiII-LnIII-NiII Complexes with Schiff Base Ligands: Synthesis, Structure, and Magnetic Properties. Molecules 2020, 25, 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102280
Georgopoulou AN, Pissas M, Psycharis V, Sanakis Y, Raptopoulou CP. Trinuclear NiII-LnIII-NiII Complexes with Schiff Base Ligands: Synthesis, Structure, and Magnetic Properties. Molecules. 2020; 25(10):2280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102280
Chicago/Turabian StyleGeorgopoulou, Anastasia N., Michael Pissas, Vassilis Psycharis, Yiannis Sanakis, and Catherine P. Raptopoulou. 2020. "Trinuclear NiII-LnIII-NiII Complexes with Schiff Base Ligands: Synthesis, Structure, and Magnetic Properties" Molecules 25, no. 10: 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25102280