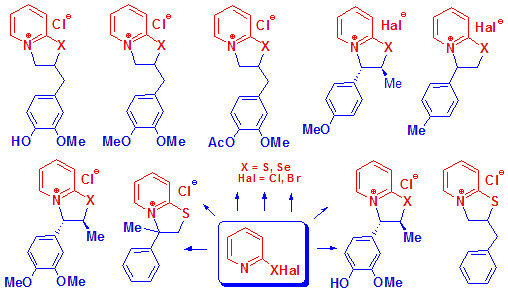
Natural Compounds and Their Structural Analogs in Regio- and Stereoselective Synthesis of New Families of Water-Soluble 2H,3H-[1,3]thia- and -Selenazolo[3,2-a]pyridin-4-ium Heterocycles by Annulation Reactions
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis of Compounds 1–18, 21–24
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, P.K.; Verma, P.K.; Tomer, I.; Mathur, G.; Dhakad, P.K. Biological Potential of Thiazole Derivatives of Synthetic Origin. J. Heterocycl. Chem. 2017, 54, 2103–2116. [Google Scholar] [CrossRef]
- Chhabria, M.T.; Patel, S.; Modi, P.; Brahmkshatriya, P.S. Thiazole: A review on chemistry, synthesis and therapeutic importance of its derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [Google Scholar] [CrossRef]
- Haddach, M.; Schwaebe, M.K.; Michaux, J.; Nagasawa, J.; O’Brien, S.E.; Whitten, J.P.; Pierre, F.; Kerdoncuff, P.; Darjania, L.; Stansfield, R.; et al. Discovery of CX-5461, the First Direct and Selective Inhibitor of RNA Polymerase I, for Cancer Therapeutics. ACS Med. Chem. Lett. 2012, 3, 602–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, J.A.D.; Kulen, A.M.; Almqvist, K.F.; Cairns, A.G.; Ponten, J.F. 2,3-Dihydro-Thiazolo[3,2-a] Pyridin-5-One Derivatives, Intermediates Thereof, and Their Use as Antibacterial Agents. WO Patent 2016075296, 19 May 2016. [Google Scholar]
- Shi, F.; Li, C.; Xia, M.; Miao, K.; Zhao, Y.; Tu, S.; Zheng, W.; Zhang, G.; Ma, N. Green chemoselective synthesis of thiazolo [3,2-a] pyridine derivatives and evaluation of their antioxidant and cytotoxic activities. Bioorg. Med. Chem. Lett. 2009, 19, 5565–5568. [Google Scholar] [CrossRef] [PubMed]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole derivatives as new chemotype targeting the HCV NS5B polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Sjogren, E.B.; Matthews, T.R. Antitrichomonal activity of mesoionic thiazolo [3,2-a]pyridines. J. Med. Chem. 1985, 28, 1673–1679. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Five-membered ring systems. Coord. Chem. Rev. 2016, 312, 149–177. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Six-membered ring systems. Coord. Chem. Rev. 2017, 330, 110–126. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Q.; Jiang, X. Transition-Metal-Free Diarylannulated Sulfide and Selenide Construction via Radical/Anion-Mediated Sulfur–Iodine and Selenium–Iodine Exchange. Org. Lett. 2016, 18, 5756–5759. [Google Scholar] [CrossRef]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Rafique, J.; Canto, R.F.S.; Saba, S.; Barbosa, F.A.R.; Braga, A.L. Recent Advances in the Synthesis of Biologically Relevant Selenium-containing 5-Membered Heterocycles. Curr. Org. Chem. 2016, 20, 166–188. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part, B. Anti-infective and anticancer compounds. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Gladyshev, V.N.; Hatfield, D.L. Selenocysteine-Containing Proteins in Mammals. J. Biomed. Sci. 1999, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef]
- Organoselenium Chemistry: Between Synthesis and Biochemistry; Santi, C. (Ed.) Bentham Science Publishers: Sharjah, UAE, 2014; p. 563. [Google Scholar]
- Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Woollins, J.D., Laitinen, R.S., Eds.; Springer: Heidelberg, Germany, 2011; p. 334. [Google Scholar]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. New methodology of nucleophilic substitution at three different centers of a seleniranium intermediate in reactions of 2-bromomethyl-1,3-thiaselenole with mercapto benzazoles. New J. Chem. 2019, 43, 11189–11199. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Potapov, V.A.; Penzik, M.V.; Makhaeva, N.A.; Albanov, A.I. Regio-and stereoselective synthesis of a novel family of unsaturated compounds with the S–Se bond and their cyclization to 2,3-dihydro-1,4-thiaselenines. Synthesis 2019, 51, 1832–1840. [Google Scholar] [CrossRef] [Green Version]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Kashik, A.S. Reactions of selenium and tellurium metals with phenylacetylene in 3-phase catalytical systems. Tetrahedron Lett. 1989, 30, 613–616. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Reaction of selenium dichloride with divinyl selenide. Russ. J. Org. Chem. 2008, 44, 1556–1557. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Compd. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Lyssenko, K.A.; Musalov, M.V.; Finn, M.G. Transannular Addition of Selenium Dichloride and Dibromide to 1,5-Cyclooctadiene: Synthesis of 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and Their Complexes with Selenium Dihalides. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkene. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, T.N.; Savikhina, E.V. Heterocyclization in the reaction of pyridine-2-selanyl chloride with styrene. Chem. Heterocycl. Comp. 2007, 43, 525–526. [Google Scholar] [CrossRef]
- Borisov, A.V.; Osmanov, V.K.; Borisova, G.N.; Matsulevich, Z.V.; Fukin, G.K. Synthesis of condensed sulfur- and nitrogen-containing heterocycles via polar cycloaddition of hetarene sulfenyl chlorides to a C–C multiple bond. Mendeleev Commun. 2009, 19, 49–51. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, G.N.; Mammadova, G.Z.; Maharramov, A.M.; Khrustalev, V.N. Cycloaddition of di (2-pyridyl) diselenide to styrene activated with antimony pentachloride. Russ. Chem. Bull. 2011, 60, 2057–2062. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, G.N.; Mammadova, G.Z.; Maharramov, A.M.; Khrustalev, V.N. Sulfenyl halides in the synthesis of heterocycles. 4*. Heterocyclization in reactions of alkenes with sulfenylating reagents based on di (2-pyridyl) disulfide. Chem. Heterocycl. Comp. 2012, 48, 1098–1104. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, G.N. Synthesis of 2,3-dihydroselenazolo[3,2-a]pyridinium salts based on reactions of pyridine-2-selanyl chloride with alkenes and dienes. Chem. Heterocycl. Comp. 2012, 48, 492–496. [Google Scholar] [CrossRef]
- Potapov, V.A.; Malinovich, D.A.; Amosova, S.V.; Rusakov, Y.Y.; Bhasin, K.K. Reaction of 2-pyridylselenenyl bromide with divinyl selenide. Chem. Heterocycl. Comp. 2012, 48, 1129–1131. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalova, M.V.; Ishigeev, R.S.; Musalov, M.V.; Panov, V.A.; Khabibulina, A.G.; Amosova, S.V.; Bhasin, K.K. Efficient and selective syntheses of novel unsaturated chalcogen-containing pyridine derivatives. Tetrahedron Lett. 2016, 57, 5341–5343. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V.; Borodina, T.N. Synthesis of a novel family of water-soluble 2H,3H-[1,3]thia- and -selenazolo[3,2-a]pyridin-4-ium heterocycles by annulation reactions. Tetrahedron Lett. 2019, 60, 475–479. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Synthesis of 3-(2-oxopyrrolidin-1-yl)-2H,3H-[1,3]selenazolo[3,2-a]pyridin-4-ium chloride. Russ. J. Org. Chem. 2017, 53, 1604–1605. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Regioselective Reaction of Pyridine-2-Sulfenyl Chloride with Isoeugenole. Russ. J. Org. Chem. 2018, 54, 1262–1263. [Google Scholar]
- Samuilov, Y.D.; Gainullin, V.I.; Solov’eva, S.E.; Konovalov, A.I. Reactivity of styrenes toward electrophilic addition of phenylsulfenyl chloride. Zhurnal Organicheskoi Khimii 1988, 24, 795–803. (In Russian) [Google Scholar]
- Liotta, D.; Zima, G. An examination of the synthetic utility of phenylselenenyl chloride additions to olefins. Tetrahedron Lett. 1978, 50, 4977–4980. [Google Scholar] [CrossRef]
- Rasteikiene, L.; Greiciute, D.; Lin’kova, M.G.; Knunyants, I.L. The Addition of Sulphenyl Chlorides to Unsaturated Compounds. Russ. Chem. Rev. 1977, 46, 548–564. [Google Scholar] [CrossRef]
- Smit, V.A.; Zefirov, N.S.; Bodrikov, I.V.; Krimer, M.Z. Episulfonium ions: Myth and reality. Acc. Chem. Res. 1979, 12, 282–288. [Google Scholar] [CrossRef]
- Abu-yousef, I.A.; Harpp, D.N. New Sulfenyl Chloride Chemistry: Synthesis, Reactions and Mechanisms toward Carbon-Carbon Double Bonds. Sulfur Rep. 2003, 24, 255–282. [Google Scholar] [CrossRef]
- Denmark, S.E.; Vogler, T. Synthesis and Reactivity of Enantiomerically Enriched Thiiranium Ions. Chem. Eur. J. 2009, 15, 11737–11745. [Google Scholar] [CrossRef]
- Denmark, S.E.; Collins, W.R.; Cullen, M.D. Observation of Direct Sulfenium and Selenenium Group Transfer from Thiiranium and Seleniranium Ions to Alkenes. J. Am. Chem. Soc. 2009, 131, 3490–3492. [Google Scholar] [CrossRef]
- Denmark, S.E.; Edwards, M.G. On the Mechanism of the Selenolactonization Reaction with Selenenyl Halides. J. Org. Chem. 2006, 71, 7293–7306. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Kalyani, D.; Collins, W.R. Preparative and mechanistic studies toward the rational development of catalytic, enantioselective selenoetherification reactions. J. Am. Chem. Soc. 2010, 132, 15752–15765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musalov, M.V.; Potapov, V.A.; Kurkutov, E.O.; Musalova, M.V.; Khabibulina, A.G.; Amosova, S.V. Regioselective syntheses of bis(2-haloalkyl) selenides and dihalo[bis(2-haloalkyl)]-λ4-selanes from selenium dihalides and 1-alkenes, and the methoxyselenenylation reaction. Arkivoc 2017, iii, 365–376. [Google Scholar] [CrossRef]
- Kurkutov, E.O.; Musalov, M.V.; Potapov, V.A.; Larina, L.I.; Amosova, S.V. Rearrangements in methanolysis of bis(2-bromoalkyl) selenides. Russ. J. Org. Chem. 2016, 52, 186–191. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Santi, C.; Genovese, S. New insights into the seleniranium ion promoted cyclization of prenyl and propenylbenzene aryl ethers. Tetrahedron Lett. 2017, 58, 371–374. [Google Scholar] [CrossRef]
- Poleschner, H.; Seppelt, K. Seleniranium and Telluriranium Salts. Chem. Eur. J. 2018, 24, 17155–17161. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Lauer, R.F. Electrophilic organoselenium reagents. New route to allylic acetates and ethers. J. Org. Chem. 1974, 39, 429–430. [Google Scholar] [CrossRef]
- Antipin, R.L.; Klak, V.N.; Beloglazkina, E.K.; Zyk, N.V. Reactions of areneselenenamides with alkenes in the presence of phosphorus (V) and sulfur (IV) oxyhalides. New synthesis of β-haloalkyl selenides. Russ. J. Org. Chem. 2009, 45, 842–847. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Nelson, D.J. Alkene selenenylation: A comprehensive analysis of relative reactivities, stereochemistry and asymmetric induction, and their comparisons with sulfenylation. Beilstein J. Org. Chem. 2011, 7, 744–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cresswell, A.J.; Eey, S.T.-C.; Denmark, S.E. Catalytic, stereospecific syn-dichlorination of alkenes. Nat. Chem. 2015, 7, 146–152. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, V.A.; Ishigeev, R.S.; Shkurchenko, I.V.; Zinchenko, S.V.; Amosova, S.V. Natural Compounds and Their Structural Analogs in Regio- and Stereoselective Synthesis of New Families of Water-Soluble 2H,3H-[1,3]thia- and -Selenazolo[3,2-a]pyridin-4-ium Heterocycles by Annulation Reactions. Molecules 2020, 25, 376. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25020376
Potapov VA, Ishigeev RS, Shkurchenko IV, Zinchenko SV, Amosova SV. Natural Compounds and Their Structural Analogs in Regio- and Stereoselective Synthesis of New Families of Water-Soluble 2H,3H-[1,3]thia- and -Selenazolo[3,2-a]pyridin-4-ium Heterocycles by Annulation Reactions. Molecules. 2020; 25(2):376. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25020376
Chicago/Turabian StylePotapov, Vladimir A., Roman S. Ishigeev, Irina V. Shkurchenko, Sergey V. Zinchenko, and Svetlana V. Amosova. 2020. "Natural Compounds and Their Structural Analogs in Regio- and Stereoselective Synthesis of New Families of Water-Soluble 2H,3H-[1,3]thia- and -Selenazolo[3,2-a]pyridin-4-ium Heterocycles by Annulation Reactions" Molecules 25, no. 2: 376. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25020376