Biotechnological Transformation of Hydrocortisone into 16α-Hydroxyprednisolone by Coupling Arthrobacter simplex and Streptomyces roseochromogenes
Abstract
:1. Introduction
2. Results
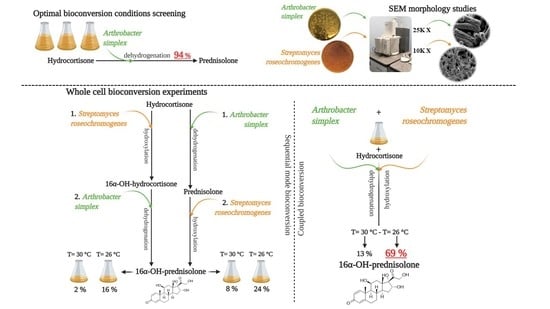
2.1. A. simplex Shake Flask Experiments
2.2. Shake Flask Experiments on Different Substrates
2.3. Whole Cell Experiments
2.3.1. Whole Cell Experiments by Using S. roseochromogenes and A. simplex in a Sequential Mode
2.3.2. Whole Cell Experiments by Using A. simplex and S. roseochromogenes in a Sequential Mode
2.3.3. Whole Cell Experiments Coupling A. simplex and S. roseochromogenes
2.4. 16α-OH-PD Purification and Characterization
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Microorganisms and Media
4.3. Shake Flask Experiments
4.3.1. A. simplex Shake Flask Experiments
4.3.2. Shake Flask Experiments on Different Substrates
4.4. Whole Cell Experiments
4.4.1. Whole Cell Experiments by Using S. roseochromogenes and A. simplex in a Sequential Mode
4.4.2. Whole Cell Experiments by Using A. simplex and S. roseochromogenes in a Sequential Mode
4.4.3. Whole Cell Experiments by Coupling A. simplex and S. roseochromogenes
4.5. Glucose Determination
4.6. Steroid Extraction and HPLC Analyses
4.7. Steroid Purification by Preparative Chromatography
4.8. NMR Analyses
4.9. Scanning Electron Microscope Analyses
4.10. Data and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahato, S.B.; Garai, S. Advances in microbial steroid biotransformation. Steroids 1997, 62, 332–345. [Google Scholar] [CrossRef]
- Holland, H.L. Recent advances in applied and mechanistic aspects of the enzymatic hydroxylation of steroids by whole-cell biocatalysts. Steroids 1999, 64, 178–186. [Google Scholar] [CrossRef]
- Fernandes, P.; Cruz, A.; Angelova, B.; Pinheiro, H.M.; Cabral, J.M.S. Microbial conversion of steroid compounds: Recent developments. Enzym. Microb. Tech. 2003, 32, 688–705. [Google Scholar] [CrossRef]
- Tong, W.-Y.; Dong, X. Microbial biotransformation: Recent developments on steroid drugs. Recent. Pat. Biotechnol. 2009, 3, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cui, L.; Wu, M.; Zhang, R.; Xie, L.; Wang, H. Transformation of prednisolone to a 20β-hydroxy prednisolone compound by Streptomyces roseochromogenes TS79. Appl. Microbiol. Biotechnol. 2011, 92, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Długoński, J.; Sedlaczek, L. Regulation of steroid 16alpha-hydroxylation in Streptomyces olivoviridis. Z. Allg. Mikrobiol. 1981, 21, 499–506. [Google Scholar] [CrossRef]
- Donova, M.V.; Egorova, O.V. Microbial steroid transformations: Current state and prospects. Appl. Microbiol. Biotechnol. 2012, 94, 1423–1447. [Google Scholar] [CrossRef]
- Donova, M.V. Transformation of steroids by actinobacteria: A review. Appl. Biochem. Microbiol. 2007, 43, 1–14. [Google Scholar] [CrossRef]
- Imirzalioglu, C.; Hain, T.; Hossain, H.; Chakraborty, T.; Domann, E. Erythema caused by a localised skin infection with Arthrobacter mysorens. BMC Infect. Dis. 2010, 10, 352. [Google Scholar] [CrossRef]
- Arinbasarova, A.Y.; Karpov, A.V.; Fokina, V.V.; Medentsev, A.G.; Koshcheyenko, K.A. Kinetic characteristics of 1-en-dehydrogenation of 6α-methylhydrocortisone by cells of Arthrobacter globiformis 193. Enzym. Microb. Tech. 1996, 19, 501–506. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, C.; Wang, M.; Lu, Y.; Wang, Z. Transformation of cortisone acetate using immobilized Arthrobacter simplex cells. Chin. J. Biotechnol. 1998, 14, 117–123. [Google Scholar]
- Luo, J.; Ning, J.; Wang, Y.; Cheng, Y.; Zheng, Y.; Shen, Y.; Wang, M. The effect of ethanol on cell properties and steroid 1-en-dehydrogenation biotransformation of Arthrobacter simplex. Biotechnol. Appl. Biochem. 2014, 61, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, F.; Wang, Y.; Zhao, Q.; Wang, M. Cyclic utilization of HP-β-CD in the bioconversion of cortisone acetate by Arthrobacter simplex. Biotechnol. Lett. 2016, 38, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Marseglia, M.; De Castro, C.; Diana, P.; Forni, P.; Parrilli, M.; De Rosa, M.; Schiraldi, C. Biotechnological transformation of hydrocortisone to 16α-hydroxy hydrocortisone by Streptomyces roseochromogenes. Appl. Microbiol. Biotechnol. 2014, 98, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Marseglia, M.; Diana, P.; Borzacchiello, M.G.; Finamore, R.; Vitiello, M.; D’Agostino, A.; De Rosa, M.; Schiraldi, C. Advances in the 16α-hydroxy transformation of hydrocortisone by Streptomyces roseochromogenes. Process Biochem. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Mazumder, T.K.; Sonomoto, K.; Tanaka, A.; Fukui, S. Sequential conversion of cortexolone to prednisolone by immobilized mycelia of Curvularia lunata and immobilized cells of Arthrobacter simplex. Appl. Microbiol. Biotechnol. 1985, 21, 154–161. [Google Scholar] [CrossRef]
- Yoshida, T.; Taguchi, H.; Kulprecha, S.; Nilubol, N. Kinetics and optimization of steroid transformation in a mixed culture. In Advances in Biotechnology Volume III, Fermentation Products: Proceedings of the Sixth International Fermentation Symposium; Vezina, C., Singh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 501–506. [Google Scholar]
- Draco, A.B. Epimers of budenoside and related corticosteroids. Acta Pharm. Suec. 1987, 24, 97–114. [Google Scholar]
- Ezaki, M.; Iwami, M.; Yamashita, M.; Komori, T.; Umehara, K.; Imanaka, H. Biphenomycin A production by a mixed culture. Appl. Environ. Microbiol. 1992, 58, 3879–3882. [Google Scholar] [CrossRef] [Green Version]
- Ezaki, M.; Shigematsu, N.; Yamashita, M.; Komori, T.; Umehara, K.; Imanaka, H. Biphenomycin C, a precursor of biphenomycin A in mixed culture. J. Antibiot. 1993, 46, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Médici, R.; Iribarren, A.M.; Lewkowicz, E.S. Synthesis of 9-beta-d-arabinofuranosylguanine by combined use of two whole cell biocatalysts. Bioorg. Med. Chem. Lett. 2009, 19, 4210–4212. [Google Scholar] [CrossRef]
- Lee, B.K.; Brown, W.E.; Ryu, D.Y.; Thoma, R.W. Sequential 11α-hydroxylation and 1-dehydrogenation of 16α-hydroxycortexolone. Biotechnol. Bioeng. 1971, 13, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Jayaveera, K.N.; Sreenivasulu, V. Pharmaceutical Biotechnology; S. Chand Publishing: New Delhi, India, 2014; pp. 71–105. [Google Scholar]
- Berrie, J.R.; Williams, R.A.D.; Smith, K.E. Microbial transformations of steroids-XI. Progesterone transformation by Streptomyces roseochromogenes–purification and characterisation of the 16α-hydroxylase system. J. Steroid Biochem. 1999, 71, 153–165. [Google Scholar] [CrossRef]
- Berrie, J.R.; Williams, R.A.; Smith, K.E. Microbial transformations of steroids--XII. Progesterone hydroxylation profiles are modulated by post-translational modification of an electron transfer protein in Streptomyces roseochromogenes. J. Steroid Biochem. 2001, 77, 87–96. [Google Scholar] [CrossRef]
- Sato, Y.; Oda, T.; Inoue, J.; Kunugi, M.; Suzuki, K.T. Stereochemistry of Microbial Hydrogenation of (-)-α-Santonin to (+)-1,2-Dihydro-α-santonin by Streptomyces cinereocrocatus NRRL 3443. Chem. Pharm. Bull. 1984, 32, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Ensign, J.C.; Wolfe, R.S. Nutritional control of morphogenesis in Arthrobacter crystallopietes. J. Bacteriol. 1964, 87, 924–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Liang, J.; Li, H.; Wang, M. Hydroxypropyl-β-cyclodextrin-mediated alterations in cell permeability, lipid and protein profiles of steroid-transforming Arthrobacter simplex. Appl. Microbiol. Biotechnol. 2015, 99, 387–397. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, L.; Liang, J.; Tang, R.; Wang, M. Effects of two kinds of imidazolium-based ionic liquids on the characteristics of steroid-transformation Arthrobacter simplex. Microb. Cell Fact. 2016, 15, 118. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Song, Z.; Ning, J.; Cheng, Y.; Wang, Y.; Cui, F.; Shen, Y.; Wang, M. The ethanol-induced global alteration in Arthrobacter simplex and its mutants with enhanced ethanol tolerance. Appl. Microbiol. Biotechnol. 2018, 102, 9331–9350. [Google Scholar] [CrossRef]
- Kofronová, O.; Nguyen, L.D.; Weiser, J.; Benada, O. Streptomycetes cultured on glass beads: Sample preparation for SEM. Microsc. Res. Tech. 2002, 58, 111–113. [Google Scholar] [CrossRef]
- Kumar, V.; Bharti, A.; Gusain, O.; Bisht, G.S. Scanning electron microscopy of Streptomyces without use of any chemical fixatives. Scanning 2011, 33, 446–449. [Google Scholar] [CrossRef]
- Ng, I.-S.; Ye, C.; Zhang, Z.; Lu, Y.; Jing, K. Daptomycin antibiotic production processes in fed-batch fermentation by Streptomyces roseosporus NRRL11379 with precursor effect and medium optimization. Bioprocess Biosyst. Eng. 2014, 37, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Palazzotto, E.; Gallo, G.; Renzone, G.; Giardina, A.; Sutera, A.; Silva, J.; Vocat, C.; Botta, L.; Scaloni, A.; Puglia, A.M. TrpM, a Small Protein Modulating Tryptophan Biosynthesis and Morpho-Physiological Differentiation in Streptomyces coelicolor A3(2). PLoS ONE 2016, 11, e0163422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmos, E.; Mehmood, N.; Haj Husein, L.; Goergen, J.-L.; Fick, M.; Delaunay, S. Effects of bioreactor hydrodynamics on the physiology of Streptomyces. Bioprocess Biosyst. Eng. 2013, 36, 259–272. [Google Scholar] [CrossRef]
- Del Sol, R.; Armstrong, I.; Wright, C.; Dyson, P. Characterization of changes to the cell surface during the life cycle of Streptomyces coelicolor: Atomic force microscopy of living cells. J. Bacteriol. 2007, 189, 2219–2225. [Google Scholar] [CrossRef] [Green Version]
- Restaino, O.F.; di Lauro, I.; Cimini, D.; Carlino, E.; De Rosa, M.; Schiraldi, C. Monosaccharide precursors for boosting chondroitin-like capsular polysaccharide production. Appl. Microbiol. Biotechnol. 2013, 97, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds hydrocortisone and prednisolone are available from the authors. |







| Position | δC | Type | δH (J in Hz) | COSY | HMBC (H→C) | H2BC (H→C) |
|---|---|---|---|---|---|---|
| 1 | 159.9 | CH | 7.45 d (9.9) | 2 | 3, 5, 6, 9, 10 | 2 |
| 2 | 127.9 | CH | 6.25 d (9.9) | 1 | 5, 10 | 1 |
| 3 | 188.9 | C | - | - | - | - |
| 4 | 122.6 | CH | 6.00 s | - | 2, 6, 10 | - |
| 5 | 174.6 | C | - | - | - | - |
| 6 | 33.1 | CH2 | 2.37 dd (9.3, 5.1) | 7 | 4, 5, 7, 8, 10 | 7 |
| 2.64 ddd (9.3, 4.5, 1.0) | 7 | 4, 5, 7, 8, 10 | 7 | |||
| 7 | 35.5 | CH2 | 1.17 m | 6 | 6, 10, 14 | 6, 8 |
| 2.09 m | 6, 8 | 6, 10, 14 | 6, 8 | |||
| 8 | 32.3 | CH | 2.13 ov | 9 | 10, 14 | 9, 7, 14 |
| 9 | 57.2 | CH | 1.03 dd (11.0, 3.5) | 8, 11 | 7, 9, 10, 11, 14, 19 | 8, 11 |
| 10 | 46.1 | C | - | - | - | - |
| 11 | 70.6 | CH | 4.38 m | 9, 12 | 9, 12 | 9, 12 |
| 12 | 40.9 | CH2 | 1.56 m | 11 | 11 | 11 |
| 2.03 m | 11 | 11 | 11 | |||
| 13 | 48.9 | C | - | - | - | - |
| 14 | 51.6 | CH | 1.92 ov | 15 | 9, 15, 17 | 9, 15 |
| 15 | 35.2 | CH2 | 1.52 ov | 14, 16 | 14, 16, 17 | 14, 16 |
| 1.93 m | 16 | 14, 16, 17 | 14, 16 | |||
| 16 | 73.5 | CH2 | 4.92 m | 15 | 14, 20 | 15 |
| 17 | 89.3 | C | - | - | - | - |
| 18 | 17.8 | CH3 | 0.99 s | - | 12, 13, 14, 17 | - |
| 19 | 21.6 | CH3 | 1.49 s | - | 1, 5, 9, 10 | - |
| 20 | 213.0 | C | - | - | - | - |
| 21 | 68.2 | CH2 | 4.26 d | 21 | 20 | - |
| 4.62 d | 20 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restaino, O.F.; Barbuto Ferraiuolo, S.; Perna, A.; Cammarota, M.; Borzacchiello, M.G.; Fiorentino, A.; Schiraldi, C. Biotechnological Transformation of Hydrocortisone into 16α-Hydroxyprednisolone by Coupling Arthrobacter simplex and Streptomyces roseochromogenes. Molecules 2020, 25, 4912. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25214912
Restaino OF, Barbuto Ferraiuolo S, Perna A, Cammarota M, Borzacchiello MG, Fiorentino A, Schiraldi C. Biotechnological Transformation of Hydrocortisone into 16α-Hydroxyprednisolone by Coupling Arthrobacter simplex and Streptomyces roseochromogenes. Molecules. 2020; 25(21):4912. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25214912
Chicago/Turabian StyleRestaino, Odile Francesca, Simona Barbuto Ferraiuolo, Addolorata Perna, Marcella Cammarota, Maria Giovanna Borzacchiello, Antonio Fiorentino, and Chiara Schiraldi. 2020. "Biotechnological Transformation of Hydrocortisone into 16α-Hydroxyprednisolone by Coupling Arthrobacter simplex and Streptomyces roseochromogenes" Molecules 25, no. 21: 4912. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25214912