Aggregate-State Effects in the Atomistic Modeling of Organic Materials for Electrochemical Energy Conversion and Storage Devices: A Perspective
Abstract
:1. Introduction
2. Effect of Aggregation When Modeling Optoelectronic Properties
2.1. Absorption Spectra and Band Alignment
2.2. Charge Transport
3. Effect of Aggregate State When Modeling Organic Battery Materials
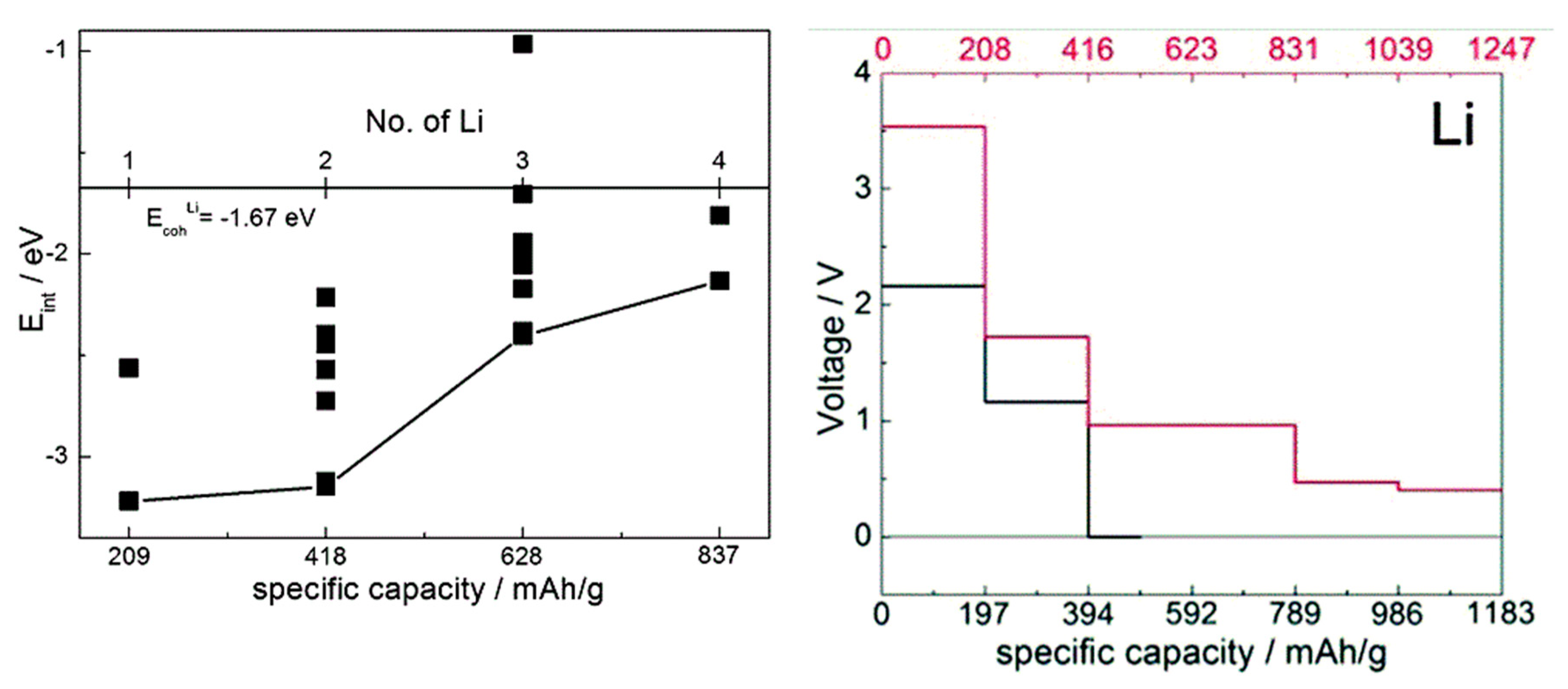
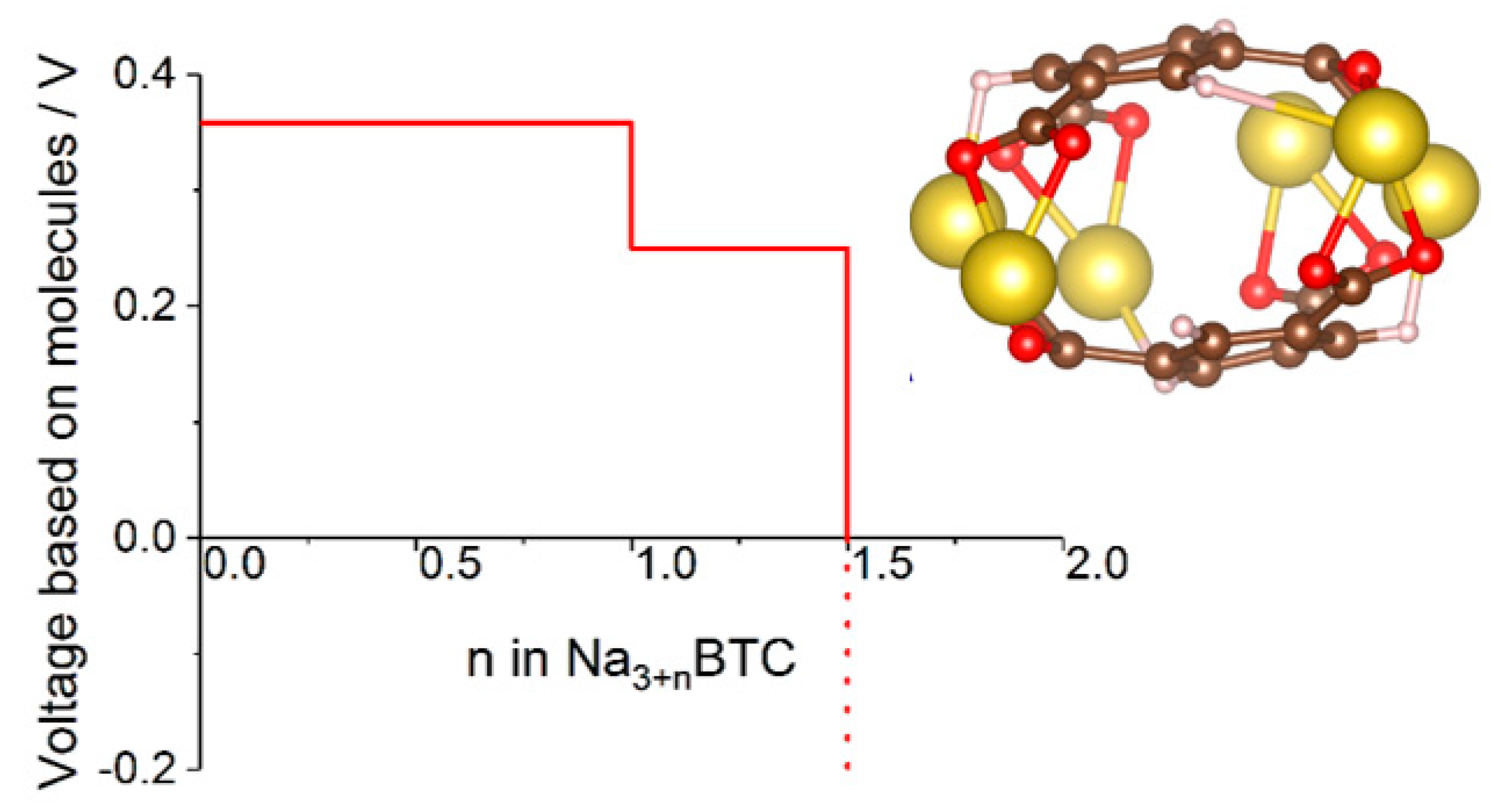
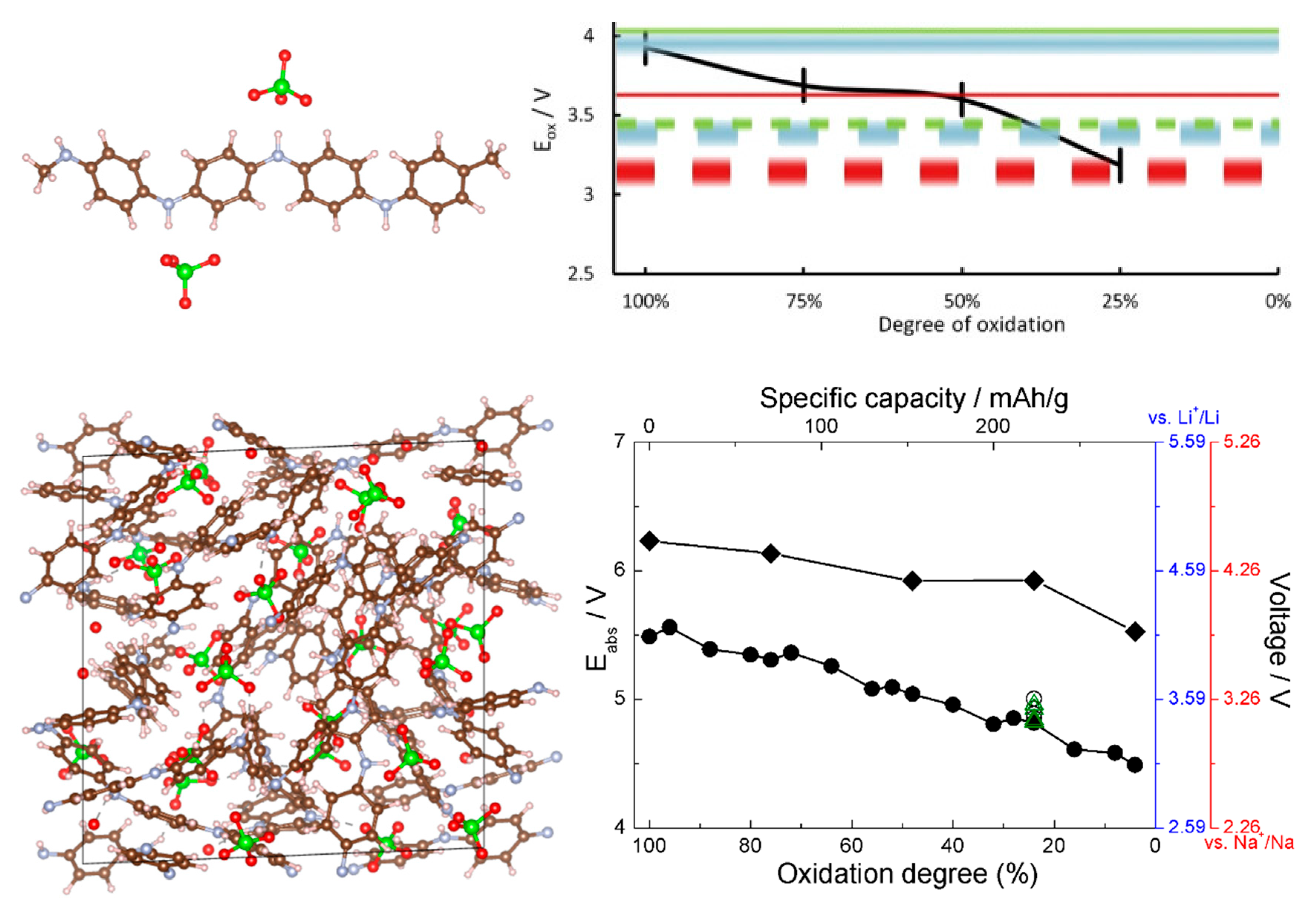
3.1. Insertion-Type Materials
3.2. p-Type Materials
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CPU | central processing unit |
| DFT | density functional theory |
| DFTB | density functional tight binding |
| DOS | density of states |
| GGA | generalized gradient approximation |
| HOMO | highest occupied molecular orbital |
| LUMO | lowest unoccupied molecular orbital |
| MD | molecular dynamics |
| MOF | metal-organic framework |
| OLED | organic light-emitting diode |
| OSC | organic solar cell |
| PANI | polyaniline |
| PLED | perovskite light-emitting diode |
| PSC | perovskite solar cell |
| TCNE | tetracyanoethylene |
| TD | time-dependent |
| UV-VIS | ultraviolet and visible |
| vdW | van der Waals |
| XRD | X-ray diffraction |
References
- Inganäs, O. Organic photovoltaics over three decades. Adv. Mater. 2018, 30, 1800388. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide perovskite photovoltaics: Background, status, and future prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Van Le, Q.; Jang, H.W.; Kim, S.Y. Recent advances toward high-efficiency halide perovskite light-emitting diodes: Review and perspective. Small Methods 2018, 2, 1700419. [Google Scholar] [CrossRef]
- Shea, J.J.; Luo, C. Organic electrode materials for metal ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 5361–5380. [Google Scholar] [CrossRef]
- Pham, H.D.; Li, X.Q.; Li, W.H.; Manzhos, S.; Kyaw, A.K.; Sonar, P. Organic interfacial materials for perovskite-based optoelectronic devices. Energy Env. Sci. 2019, 12, 1177–1209. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.-D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef] [PubMed]
- Sabeo, S.; Pulay, P. Local treatment of electron correlation. Annu. Rev. Phys. Chem. 1991, 44, 213–236. [Google Scholar] [CrossRef]
- Bartlett, R.J. Coupled-cluster approach to molecular structure and spectra: A step toward predictive quantum chemistry. J. Phys. Chem. 1989, 93, 1697–1708. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Arabnejad, S.; Manzhos, S.; He, C.; Shim, V.P.W. Shear-induced conformation change in α-crystalline Nylon6. Appl. Phys. Lett. 2014, 105, 221910. [Google Scholar] [CrossRef]
- Arabnejad, S.; Yamashita, K.; Manzhos, S. Defects in crystalline PVDF: A Density Functional Theory-Density Functional Tight Binding study. Phys. Chem. Chem. Phys. 2017, 19, 7560–7567. [Google Scholar] [CrossRef]
- Arabnejad, S.; Manzhos, S. Defects in alpha and gamma crystalline nylon6: A computational study. AIP Adv. 2015, 5, 107123. [Google Scholar] [CrossRef] [Green Version]
- Onoda, M.; Manda, Y.; Yokoyama, M.; Sugimoto, R.; Yoshino, K. A photoelectron emission study of polythiophene derivatives. J. Phys. Condens. Matter 1989, 1, 3859. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Yuan, L.; He, C.; Lu, K.; Wei, Z. Effects of shortened alkyl Chains on solution-processable small molecules with oxo-alkylated nitrile end-capped acceptors for high-performance organic solar cells. Adv. Energy Mater. 2014, 4, 1400538. [Google Scholar] [CrossRef]
- Ponnappa, S.P.; Arumugam, S.; Manzhos, S.; MacLeod, J.; Spratt, H.J.; O’Mullane, A.P.; Sonar, P. Investigation of thiophene flanked diketopyrrolopyrrole monomers with straight and branched alkyl chains and their electropolymerization study. J. Mater. Res. 2017, 32, 2707–2718. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260. [Google Scholar] [CrossRef]
- Gaus, M.; Cui, Q.; Elstner, M. DFTB3: Extension of the Self-Consistent-Charge Density-Functional Tight-Binding Method (SCC-DFTB). J. Chem. Theory Comput. 2011, 7, 931–948. [Google Scholar] [CrossRef] [Green Version]
- Niehaus, T.A.; Suhai, S.; Della Sala, F.; Lugli, P.; Elstner, M.; Seifert, G.; Frauenheim, T. Tight-binding approach to time-dependent density-functional response theory. Phys. Rev. B 2001, 63, 085108. [Google Scholar] [CrossRef] [Green Version]
- Niehaus, T.A.; Heringer, D.; Torralva, B.; Frauenheim, T. Importance of electronic self-consistency in the TDDFT based treatment of nonadiabatic molecular dynamics. Eur. Phys. J. D 2005, 35, 467. [Google Scholar] [CrossRef]
- Li, W.; Kotsis, K.; Manzhos, S. Comparative Density Functional Theory and Density Functional Tight Binding study of arginine and arginine-rich cell penetrating peptide TAT adsorption on anatase TiO2. Phys. Chem. Chem. Phys. 2016, 18, 19902–19917. [Google Scholar] [CrossRef] [Green Version]
- Stöhr, M.; Van Voorhis, T.; Tkatchenko, A. Theory and practice of modeling van der Waals interactions in electronic-structure calculations. Chem. Soc. Rev. 2019, 48, 4118–4154. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical gga-type density functional constructed with a long-range dispersion correction. J. Comp. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, L.E.; Haynes, P.D. Ab initio calculations of the optical absorption spectra of C60-conjugated polymer hybrids. Phys. Chem. Chem. Phys. 2013, 15, 13024–13031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.-T.; Boschetto, G.; Krompiec, M.; Morse, G.E.; Tang, F.-L.; Skylaris, C.-K. Linear-scaling density functional simulations of the effect of crystallographic structure on the electronic and optical properties of fullerene solvates. Phys. Chem. Chem. Phys. 2017, 19, 5617–5628. [Google Scholar] [CrossRef] [Green Version]
- Sk, M.A.; Manzhos, S. Exploring the sodium storage mechanism in disodium terephthalate as anode for organic battery using density-functional theory calculations. J. Power Sources 2016, 324, 572–581. [Google Scholar] [CrossRef]
- Sk, M.A.; Manzhos, S. Sodium interaction with disodium terephthalate molecule: An ab initio study. Mrs Adv. 2016, 1, 3579–3584. [Google Scholar] [CrossRef]
- Chen, Y.; Manzhos, S. Comparative computational study of lithium and sodium insertion in van der Waals and covalent tetracyanoethylene (TCNE) -based crystals as promising materials for organic lithium and sodium ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 8874–8880. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Manzhos, S. A computational study of lithium interaction with tetracyanoethylene (TCNE) and tetracyaniquinodimethane (TCNQ) molecules. Phys. Chem. Chem. Phys. 2016, 18, 1470–1477. [Google Scholar] [CrossRef]
- Pal, A.; Lai, K.W.; Chia, Y.J.; Jeon, I.; Matsuo, Y.; Manzhos, S. Comparative Density Functional Theory–Density Functional Tight Binding Study of fullerene derivatives: Effects due to fullerene size, addends, and crystallinity on bandstructure, charge transport and optical properties. Phys. Chem. Chem. Phys. 2017, 19, 28330–28343. [Google Scholar] [CrossRef]
- Padhy, H.; Chen, Y.; Lüder, J.; Gajella, S.R.; Manzhos, S.; Balaya, P. Charge and discharge processes and sodium storage in disodium pyridine-2,5-dicarboxylate anode - insights from experiments and theory. Adv. Energy Mater. 2018, 8, 1701572. [Google Scholar] [CrossRef]
- Chen, Y.; Lueder, J.; Manzhos, S. Disodium pyridine dicarboxylate vs. disodium terephthalate as anode materials for organic Na ion batteries: Effect of molecular structure on voltage from the molecular modeling perspective. Mrs Adv. 2017, 2, 3231–3235. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-S.; Jeon, I.; Xiang, R.; Seo, S.; Lee, J.-W.; Li, C.; Pal, A.; Manzhos, S.; Goorsky, M.-S.; Yang, Y.; et al. Achieving high efficiency in solution-processed perovskite solar cells using C60/C70 mixed fullerenes. ACS Appl. Mater. Interfaces 2018, 10, 39590–39598. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Arabnejad, S.; Yamashita, K.; Manzhos, S. Influence of the aggregate state on band structure and optical properties of C60 computed with different methods. J. Chem. Phys. 2018, 148, 204301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, S.-T.; Pal, A.; Manzhos, S. Comparison of optical absorption spectra of organic molecules and aggregates computed from real frequency dependent polarizability to TD-DFT and the dipole approximation. J. Chem. Phys. 2018, 149, 044114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lueder, J.; Ng, M.F.; Sullivan, M.; Manzhos, S. Polyaniline and CN-functionalized polyaniline as organic cathodes for lithium and sodium ion batteries: A combined molecular dynamics and Density Functional Tight Binding Study in solid state. Phys. Chem. Chem. Phys. 2018, 20, 232–237. [Google Scholar] [CrossRef]
- Chen, Y.; Manzhos, S. Voltage and capacity control of polyaniline based organic cathodes: An ab initio study. J. Power Sources 2016, 336, 126–131. [Google Scholar] [CrossRef]
- Tripathi, A.; Chen, Y.; Padhy, H.; Manzhos, S.; Balaya, P. Experimental and theoretical studies of trisodium-1,3,5- benzene tricarboxylate as a low voltage anode material for sodium ion batteries. Energy Technol. 2019, 7, 1801030. [Google Scholar] [CrossRef]
- Arabnejad, S.; Pal, A.; Yamashita, K.; Manzhos, S. Effect of nuclear motion on charge transport in fullerenes: A combined Density Functional Tight Binding-Density Functional Theory investigation. Front. Energy Res. 2019, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Manzhos, S. Organic electrode materials for lithium and post-lithium batteries: An ab initio perspective on design. Curr. Opin. Green Sustain. Chem. 2019, 17, 8–14. [Google Scholar] [CrossRef]
- Liu, Q.; Chavhan, S.; Zhang, H.; Sun, H.; Brock, A.; Manzhos, S.; Chen, Y.; Feron, K.; Durrant, J.R.; Bottle, S.E.; et al. Versatility of naphthalene flanked diketopyrrolopyrrole in electronics with >23% efficiency for dopant-free perovskite solar cells. to be published.
- Kümmel, S. Charge-transfer excitations: A challenge for Time-Dependent Density Functional Theory that has been met. Adv. Energy Mater. 2017, 7, 1700440. [Google Scholar] [CrossRef] [Green Version]
- Casida, M.E. Time-dependent density-functional theory for molecules and molecular solids. J. Mol. Struct. 2009, 914, 3–18. [Google Scholar] [CrossRef]
- Manzhos, S.; Segawa, H.; Yamashita, K. Computational dye design by changing the conjugation order: Failure of LR-TDDFT to predict relative excitation energies in organic dyes differing by the position of the methine unit. Chem. Phys. Lett. 2012, 527, 51–56. [Google Scholar] [CrossRef]
- Clausius, R. Abhandlungungen über die mechanische Wärmetheorie. Friedrich Vieweg und Sohn Braunschweig 1867, 2. [Google Scholar]
- David, W.I.F.; Ibberson, R.M.; Matthewman, J.C.; Prassides, K.; Dennis, T.J.S.; Hare, J.P.; Kroto, H.W.; Taylor, R.; Walton, D.R.M. Crystal structure and bonding of ordered C60. Nature 1991, 353, 147–149. [Google Scholar] [CrossRef]
- Pfuetzner, S.; Meiss, J.; Petrich, A.; Riede, M.; Leo, K. Improved bulk heterojunction organic solar cells employing C70 fullerenes. Appl. Phys. Lett. 2009, 94, 223307. [Google Scholar] [CrossRef]
- Pavlovich, V.S.; Shpilevsky, E.M. Absorption and fluorescence spectra of C60 fullerene concentrated solutions in hexane and polystyrene at 77–300 K. J. Appl. Spectrosc. 2010, 77, 335–342. [Google Scholar] [CrossRef]
- Soldatov, A.V.; Roth, G.; Dzyabchenko, A.; Johnels, D.; Lebedkin, S.; Meingast, C.; Sundqvist, B.; Haluska, M.; Kuzmany, H. Topochemical polymerization of C70 controlled by monomer crystal packing. Science 2001, 293, 680–683. [Google Scholar] [CrossRef] [Green Version]
- Schiefer, S.; Huth, M.; Dobrinevski, A.; Nickel, B. Determination of the crystal structure of substrate-induced pentacene polymorphs in fiber structured thin films. J. Am. Chem. Soc. 2007, 129, 10316–10317. [Google Scholar] [CrossRef]
- Manzhos, S.; Komatsu, M.; Nakazaki, J.; Segawa, H.; Yamashita, K. Theoretical analysis of the solvatochromism of organic dyes differing by the conjugation sequence. J. Photon. Energy 2012, 2, 028001. [Google Scholar] [CrossRef]
- Komatsu, M.; Nakazaki, J.; Uchida, S.; Kubo, T.; Segawa, H. A donor–acceptor type organic dye connected with a quinoidal thiophene for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 3227–3232. [Google Scholar] [CrossRef]
- Ostroverkhova, O.; Shcherbyna, S.; Cooke, D.G.; Egerton, R.F.; Hegmann, F.A.; Tykwinski, R.R.; Parkin, S.R.; Anthony, J.E. Optical and transient photoconductive properties of pentacene and functionalized pentacene thin films: Dependence on film morphology. J. Appl. Phys. 2005, 98, 033701. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Nan, G.; Yang, X.; Peng, Q.; Li, Q.; Shuai, Z. Computational methods for design of organic materials with high charge mobility. Chem. Soc. Rev. 2010, 39, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, B.; Kirkpatrick, J.; Andrienko, D. Density-functional based determination of intermolecular charge transfer properties for large-scale morphologies. Phys. Chem. Chem. Phys. 2010, 12, 11103–11113. [Google Scholar] [CrossRef] [PubMed]
- Rühle, V.; Lukyanov, A.; May, F.; Schrader, M.; Vehoff, T.; Kirkpatrick, J.; Baumeier, B.; Andrienko, D. Microscopic simulations of charge transport in disordered organic semiconductors. J. Chem. Theory Comput. 2011, 7, 3335–3345. [Google Scholar] [CrossRef]
- Arntsen, C.; Reslan, R.; Hernandez, S.; Gao, Y.; Neuhauser, D. Direct delocalization for calculating electron transfer in fullerenes. Int. J. Quantum Chem. 2013, 113, 1885–1889. [Google Scholar] [CrossRef]
- Pelzer, K.M.; Vázquez-Mayagoitia, A.; Ratcliff, L.E.; Tretiak, S.; Bair, R.A.; Gray, S.K.; Van Voorhis, T.; Larsen, R.E.; Darling, S.B. Molecular dynamics and charge transport in organic semiconductors: A classical approach to modeling electron transfer. Chem. Sci. 2017, 8, 2597–2609. [Google Scholar] [CrossRef] [Green Version]
- Mauger, A.; Julien, C.; Paolella, A.; Armand, M.; Zaghib, K. Recent progress on organic electrodes materials for rechargeable batteries and supercapacitors. Materials 2019, 12, 1770. [Google Scholar] [CrossRef] [Green Version]
- Lüder, J.; Manzhos, S. First-principle insights into molecular design for high-voltage organic electrode materials for Mg based batteries. Front. Chem. 2020, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Hanyu, Y.; Honma, I. Rechargeable quasi-solid state lithium battery with organic crystalline cathode. Sci. Rep. 2012, 2, 453. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-H.; Zhang, S.-Y.; Lv, H.-Y.; Li, W.-J.; Lu, Z.; Yang, C.; Zhong, G.-H. [n]Phenacenes: Promising organic anodes for potassium-ion batteries. J. Phys. Chem. C 2020, 124, 6964–6970. [Google Scholar] [CrossRef]
- Urban, A.; Seo, D.-H.; Ceder, G. Computational understanding of Li-ion batteries. npj Comput. Mater. 2016, 2, 16002. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, W.; Zhang, C.; Wu, Y.; Yuan, Q.; Whittaker, A.K.; Zhao, X.S. Sodium-ion storage mechanism in triquinoxalinylene and a strategy for improving electrode stability. Energy Fuels 2020. in print. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cho, C.-R.; Manzhos, S. Lithium attachment to C60 and nitrogen- and boron-doped C60: A mechanistic study. Materials 2019, 12, 2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wang, Z.; Shi, Y.; Sun, P.; Xu, Y. Poorly soluble 2,6-dimethoxy-9,10-anthraquinone cathode for lithium-ion batteries: The role of electrolyte concentration. ACS Appl. Mater. Interfaces 2020, 12, 7179–7185. [Google Scholar] [CrossRef]
- Dardenne, N.; Blase, X.; Hautier, G.; Charlier, J.-C.; Rignanese, G.-M. Ab initio calculations of open-cell voltage in Li-ion organic radical batteries. J. Phys. Chem. C 2015, 119, 23373–23378. [Google Scholar] [CrossRef]
- Ratnakumar, B.V.; di Stefano, S.; Williams, R.M.; Nagasubramanian, G.; Bankston, C.P. Organic cathode materials in sodium batteries. J. Appl. Electrochem. 1990, 20, 357–364. [Google Scholar] [CrossRef]
- Lee, M.; Hong, J.; Lopez, J.; Sun, Y.; Feng, D.; Lim, K.; Chueh, W.C.; Toney, M.F.; Cui, Y.; Bao, Z. High-performance sodium–organic battery by realizing four-sodium storage in disodium rhodizonate. Nat. Energy 2017, 2, 861–868. [Google Scholar] [CrossRef]
- Lueder, J.; Legrain, F.; Chen, Y.; Manzhos, S. Doping of active electrode materials for electrochemical batteries: An electronic structure perspective. MRS Commun. 2017, 7, 523–540. [Google Scholar] [CrossRef]
- Lueder, J.; Cheow, M.H.; Manzhos, S. Understanding doping strategies in the design of organic electrode materials for Li and Na ion batteries: An electronic structure perspective. Phys. Chem. Chem. Phys. 2017, 19, 13195–13209. [Google Scholar] [CrossRef] [Green Version]
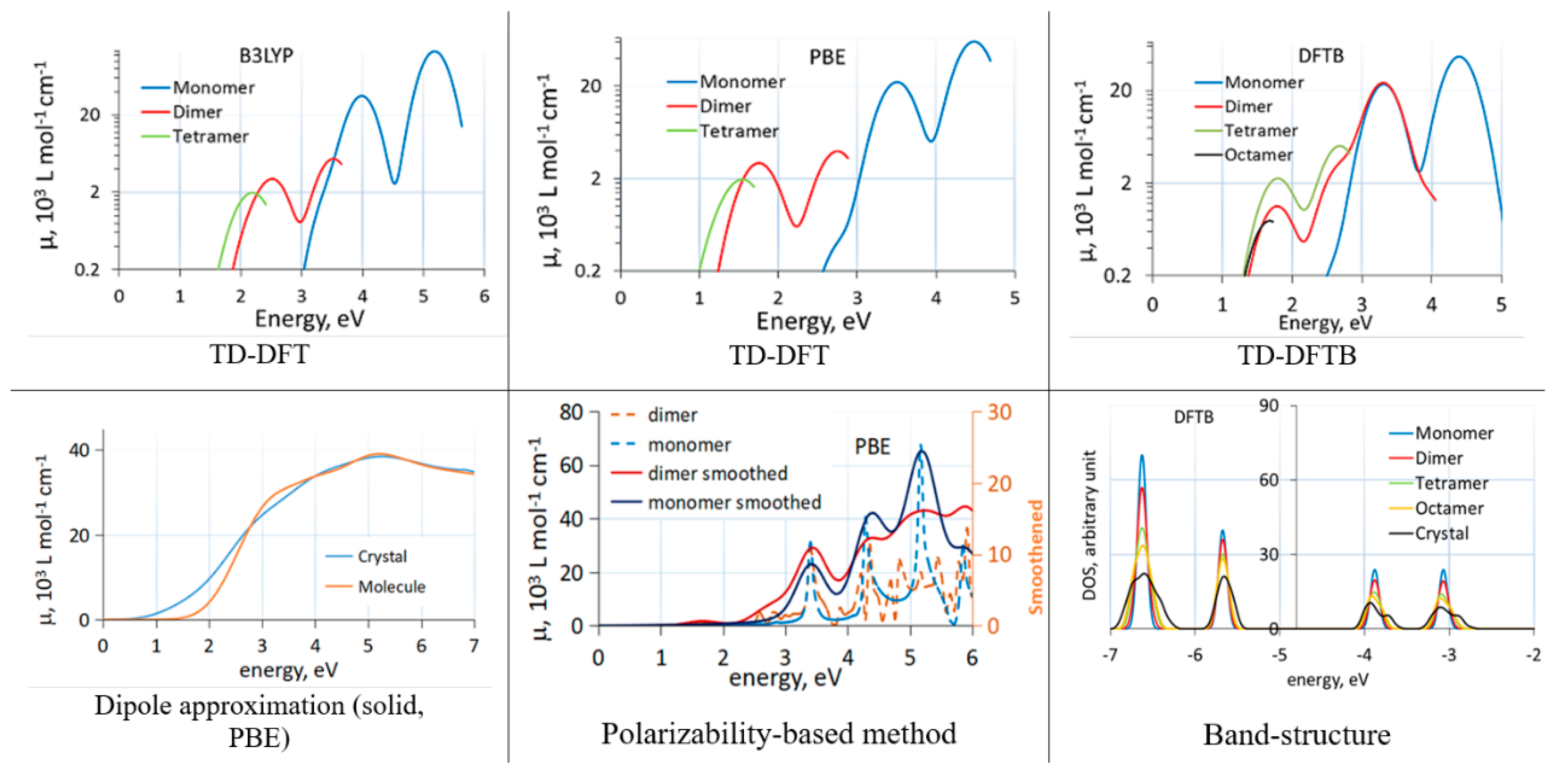


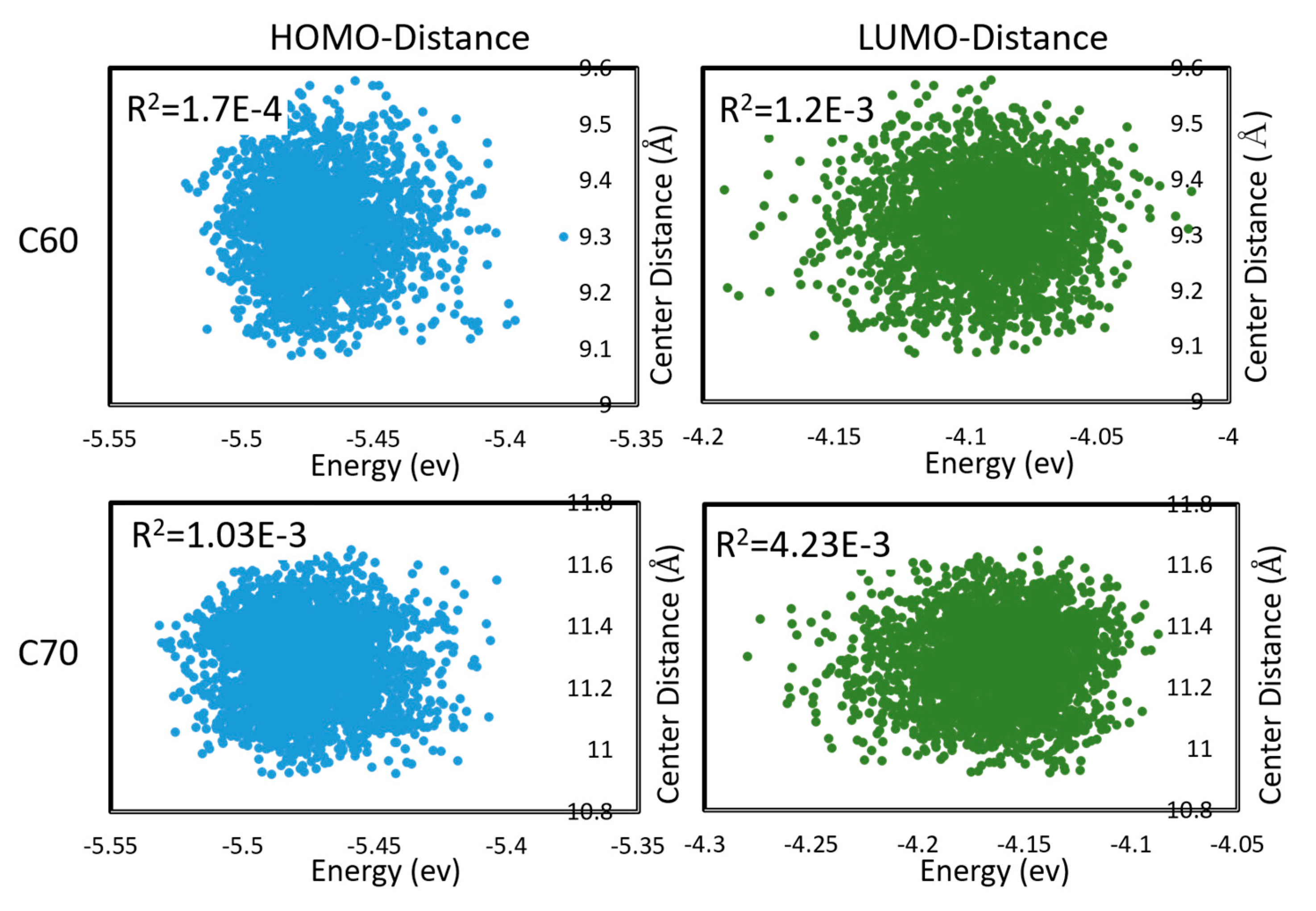
| Equil. | <…> | σ | λ | ΔGeq | ||
|---|---|---|---|---|---|---|
| LUMO | C60 | −3.98 | −4.09 | 0.023 | 0.132 | 0.15 |
| C70 | −4.04 | −4.16 | 0.027 | 0.123 | 0.15 | |
| HOMO | C60 | −5.60 | −5.47 | 0.019 | 0.167 | 0.23 |
| C70 | −5.54 | −5.48 | 0.020 | 0.138 | 0.34 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzhos, S. Aggregate-State Effects in the Atomistic Modeling of Organic Materials for Electrochemical Energy Conversion and Storage Devices: A Perspective. Molecules 2020, 25, 2233. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092233
Manzhos S. Aggregate-State Effects in the Atomistic Modeling of Organic Materials for Electrochemical Energy Conversion and Storage Devices: A Perspective. Molecules. 2020; 25(9):2233. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092233
Chicago/Turabian StyleManzhos, Sergei. 2020. "Aggregate-State Effects in the Atomistic Modeling of Organic Materials for Electrochemical Energy Conversion and Storage Devices: A Perspective" Molecules 25, no. 9: 2233. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules25092233






