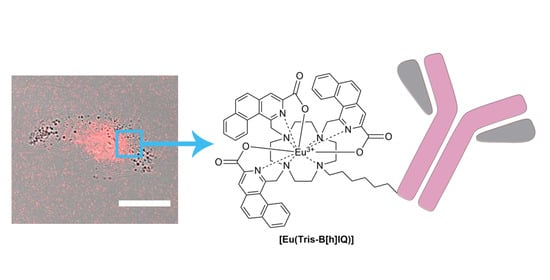
New 1,3-Disubstituted Benzo[h]Isoquinoline Cyclen-Based Ligand Platform: Synthesis, Eu3+ Multiphoton Sensitization and Imaging Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Computational Calculations
2.2. Synthetic Strategy and Results
2.3. Photophysical Characterization
2.4. Structured Illumination Imaging in the Biological Context
3. Materials and Methods
3.1. Synthetic Procedures and Characterization
3.2. Photophysical Characterization of Na[Eu(Tris-B[h]IQ)]
3.3. Conjugation of Na[Eu(Tris-B[h]IQ)] with Secondary IgG Donkey Anti Rabbit Antibody (Ab-Eu)
3.4. Cell Cultures
3.5. Immunostaining
3.6. Fluorescence Microscopy Imaging
3.7. Computational Methods and Additional Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide Probes for bioresponsive Imaging. Chem Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoroso, A.J.; Pope, S.J.A. Using Lanthanide Ions in Molecular Bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliseeva, S.V.; Bunzli, J.C. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Y. Recent Advances in the Sensitized Luminescence of Organic Europium Complexes. Coord. Chem. Rev. 2010, 254, 972–990. [Google Scholar] [CrossRef]
- Zwier, J.M.; Bazin, H.; Lamarque, L.; Mathis, G. Luminescent Lanthanides Cryptates: From the Bench to the Bedside. Inorg. Chem. 2014, 53, 1854–1866. [Google Scholar] [CrossRef]
- Afsari, H.S.; Santos, M.C.D.; Linden, S.; Chen, T.; Qiu, X.; Henegouwen, P.; Jennings, T.L.; Susumu, K.; Medintz, I.L.; Hildebrandt, N.; et al. Time-gated FRET Nanoassemblies for Rapid and Sensitive Intra- and Extracellular Fluorescence Imaging. Sci. Adv. 2016, 2, e1600265. [Google Scholar] [CrossRef] [Green Version]
- Geissler, D.; Linden, S.; Liermann, K.; Wegner, K.D.; Charbonniere, L.J.; Hildebrandt, N. Lanthanides and Quantum Dots as Forster Resonance Energy Transfer Agents for Diagnostic and Cellular Imaging. Inorg. Chem. 2014, 53, 1824–1838. [Google Scholar] [CrossRef]
- Grichine, A.; Haefele, A.; Pascal, S.; Duperray, A.; Michel, R.; Andraud, C.; Maury, O. Millisecond Lifetime Imaging with a Europium Complex Using a Commercial Confocal Microscope Under One or Two-Photon Excitation. Chem. Sci. 2014, 5, 3475–3485. [Google Scholar] [CrossRef]
- Bui, A.T.; Beyler, M.; Liao, Y.Y.; Grichine, A.; Duperray, A.; Mulatier, J.C.; Le Guennic, B.; Andraud, C.; Maury, O.; Tripier, R. Cationic Two-Photon Lanthanide Bioprobes Able to Accumulate in Live Cells. Inorg. Chem. 2016, 55, 7020–7025. [Google Scholar] [CrossRef]
- Hanaoka, K.; Kikuchi, K.; Kobayashi, S.; Nagano, T. Time-Resolved Long-Lived Luminescence Imaging Method Employing Luminescent Lanthanide Probes with a New Microscopy System. J. Am. Chem. Soc. 2007, 129, 13502–13509. [Google Scholar] [CrossRef]
- Ramshesh, V.K.; Lemasters, J.J. Pinhole Shifting Lifetime Imaging Microscopy. J. Biomed. Opt. 2008, 13, 10–64001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chudakov, D.M.; Matz, M.V.; Lukyanov, S.; Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010, 90, 1103–1163. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Latva, M.; Takalo, H.; Mukkala, V.M.; Matachescu, C.; RodriguezUbis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y.; Nakanishi, T. Effective photosensitized, electrosensitized, and mechanosensitized luminescence of lanthanide complexes. NPG Asia. Mater. 2018, 10, 52–70. [Google Scholar] [CrossRef] [Green Version]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; Vandertol, E.B.; Verhoeven, J.W. New Sensitizer-Modified Calix[4]arenes Enabling Near-UV Excitation of Complexed Luminescent Lanthanide Ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.M.; Ma, Y.; Fu, L.M.; Hao, R.; Shao, G.S.; Tang, M.X.; Zhang, J.P.; Wang, Y. A europium complex with enhanced long-wavelength sensitized luminescent properties. Phys. Chem. Chem. Phys. 2010, 12, 3195–3202. [Google Scholar] [CrossRef]
- Wei, C.; Wei, H.B.; Yan, W.B.; Zhao, Z.F.; Cai, Z.L.; Sun, B.X.; Meng, Z.S.; Liu, Z.W.; Bian, Z.Q.; Huang, C.H. Water-Soluble and Highly Luminescent Europium(III) Complexes with Favorable Photostability and Sensitive pH Response Behavior. Inorg. Chem. 2016, 55, 10645–10653. [Google Scholar] [CrossRef]
- Parker, D. Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord. Chem. Rev. 2000, 205, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Caille, F.; Bonnet, C.S.; Buron, F.; Villette, S.; Helm, L.; Petoud, S.; Suzenet, F.; Toth, E. Isoquinoline-based lanthanide complexes: Bright NIR optical probes and efficient MRI agents. Inorg. Chem. 2012, 51, 2522–2532. [Google Scholar] [CrossRef]
- Vithanarachchi, S.M.; Kovacs, D.; Borbas, K.E. Synthesis and photophysical characterization of luminescent lanthanide complexes of nucleotide-functionalized cyclen- and dipicolinate-based ligands. Inorg. Chim. Acta 2017, 460, 148–158. [Google Scholar] [CrossRef]
- Signore, G.; Nifosi, R.; Albertazzi, L.; Bizzarri, R. A novel coumarin fluorescent sensor to probe polarity around biomolecules. J. Biomed. Nanotechnol. 2009, 5, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Signore, G.; Nifosi, R.; Albertazzi, L.; Storti, B.; Bizzarri, R. Polarity-Sensitive Coumarins Tailored to Live Cell Imaging. J. Am. Chem. Soc. 2010, 132, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Signore, G.; Abbandonato, G.; Storti, B.; Stockl, M.; Subramaniam, V.; Bizzarri, R. Imaging the static dielectric constant in vitro and in living cells by a bioconjugable GFP chromophore analog. Chem. Comm. 2013, 49, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Abbandonato, G.; Polli, D.; Viola, D.; Cerullo, G.; Storti, B.; Cardarelli, F.; Salomone, F.; Nifosi, R.; Signore, G.; Bizzarri, R. Simultaneous Detection of Local Polarizability and Viscosity by a Single Fluorescent Probe in Cells. Biophys. J. 2018, 114, 2212–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storti, B.; Margheritis, E.; Abbandonato, G.; Domenichini, G.; Dreier, J.; Testa, I.; Garau, G.; Nifosi, R.; Bizzarri, R. Role of Gln222 in Photoswitching of Aequorea Fluorescent Proteins: A Twisting and H-Bonding Affair? ACS Chem. Biol. 2018, 13, 2082–2093. [Google Scholar] [CrossRef]
- Abbandonato, G.; Storti, B.; Tonazzini, I.; Stockl, M.; Subramaniam, V.; Montis, C.; Nifosi, R.; Cecchini, M.; Signore, G.; Bizzarri, R. Lipid-Conjugated Rigidochromic Probe Discloses Membrane Alteration in Model Cells of Krabbe Disease. Biophys. J. 2019, 116, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Mertz, J. Optical sectioning microscopy with planar or structured illumination. Nat. Methods 2011, 8, 811–819. [Google Scholar] [CrossRef]
- Neil, M.A.A.; Juskaitis, R.; Wilson, T. Method of obtaining optical sectioning by using structured light in a conventional miscroscope. Optic. Lett. 1997, 22, 1905–1907. [Google Scholar] [CrossRef]
- Greco, C.; Moro, G.; Bertini, L.; Biczysko, M.; Barone, V.; Cosentino, U. Computational Investigation on the Spectroscopic Properties of Thiophene Based Europium β-Diketonate Complexes. J. Chem. Theory Comput. 2014, 10, 767–777. [Google Scholar] [CrossRef]
- Chin, K.O.A.; Morrow, J.R.; Lake, C.H.; Churchill, M.R. Synthesis and solution properties of lanthanum(III), europium(III), and lutetium(III) THP complexes and an x-ray diffraction study of a crystal containing four stereoisomers of a europium(III) THP complex (THP = 1,4,7,10-tetrakis(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane). Methyl groups impart rigidity to S,S,S,S-THP macrocyclic complexes. Inorg. Chem. 1994, 33, 656–664. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Jacquemin, D.; Wathelet, V.; Perpete, E.A.; Adamo, C. Extensive TD-DFT Benchmark: Singlet-Excited States of Organic Molecules. J. Chem. Theory. Comput. 2009, 5, 2420–2435. [Google Scholar] [CrossRef] [PubMed]
- Dix, I.; Doll, C.; Hopf, H.; Jones, P.G. Model reactions for the synthesis of azacorannulenes and related heteroaromatic compounds. Eur. J. Org. Chem. 2002, 2547–2556. [Google Scholar] [CrossRef]
- Hewlins, M.J.E.; Salter, R. Preparation of Polycyclic Azaarenes by an Extended Pomeranz-Fritsch Procedure. Synthesis 2007, 14, 2157–2163. [Google Scholar] [CrossRef]
- Mamane, V.; Louerat, F.; Lehl, J.; Abboud, M.; Fort, Y. A general and efficient method for the synthesis of benzo-(iso)quinoline derivatives. Tetrahedron 2008, 64, 10699–10705. [Google Scholar] [CrossRef]
- Morimoto, K.; Hirano, K.; Satoh, T.; Miura, M. Rhodium-catalyzed Oxidative Coupling of Benzylamines with Alkynes through Dehydrogenation and Dehydrogenative Cyclization. Chem. Lett. 2011, 40, 600–602. [Google Scholar] [CrossRef]
- Caille, F.; Buron, F.; Toth, E.; Suzenet, F. Efficient Access to C1- and C3-Functionalized Isoquinolines: Towards Potential Lanthanide Ligands. Eur. J. Org. Chem. 2011, 11, 2120–2127. [Google Scholar] [CrossRef]
- Yoo, J.S.; Reichert, D.E.; Welch, M.J. Comparative in Vivo Behavior Studies of Cyclen-Based Copper-64 Complexes: Regioselective Synthesis, X-ray Structure, Radiochemistry, log P, and Biodistribution. J. Med. Chem. 2004, 47, 6625–6637. [Google Scholar] [CrossRef]
- Leon-Rodriguez, L.M.; De Kovacs, Z.; Esqueda-Oliva, A.C.; Miranda-Vera, A.D. Highly regioselective N-trans symmetrical diprotection of cyclen. Tetrahedron Lett. 2006, 47, 6937–6940. [Google Scholar] [CrossRef]
- Massue, J.; Plush, S.E.; Bonnet, C.S.; Moore, D.A.; Gunnlaugsson, T. Selective mono N-alkylations of cyclen in one step syntheses. Tetrahedron Lett. 2007, 48, 8052–8055. [Google Scholar] [CrossRef]
- Suchy, M.; Hudson, R.H.E. Synthetic Strategies Toward N-Functionalized Cyclens. Eur. J. Org. Chem. 2008, 29, 4847–4865. [Google Scholar] [CrossRef]
- Cakic, N.; Gunduz, S.; Rengarasu, R.; Angelovski, G. Synthetic strategies for preparation of cyclen-based MRI contrast agents. Tetrahedron Lett. 2015, 56, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Aebischer, A.; Gumy, F.; Bunzli, J.C.G. Intrinsic quantum yields and radiative lifetimes of lanthanide tris(dipicolinates). Phys. Chem. Chem. Phys. 2009, 11, 1346–1353. [Google Scholar] [CrossRef]
- Storti, B.; Civita, S.; Faraci, P.; Maroni, G.; Krishnan, I.; Levantini, E.; Bizzarri, R. Fluorescence imaging of biochemical relationship between ubiquitinated histone 2A and Polycomb complex protein BMI1. Biophys. Chem. 2019, 253, 106225. [Google Scholar] [CrossRef]
- Schuettengruber, B.; Bourbon, H.M.; Di Croce, L.; Cavalli, G. Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 2017, 171, 34–57. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, R.; Mustafi, S.B.; Street, M.; Dey, A.; Dwivedi, S.K. Bmi-1: At the crossroads of physiological and pathological biology. Genes Dis. 2015, 2, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.X.; Bombard, J.; Cintron, K.; Sheedy, J.; Weetall, M.L.; Davis, T.W. BMI1 as a novel target for drug discovery in cancer. J. Cell. Biochem. 2011, 112, 2729–2741. [Google Scholar] [CrossRef]
- Xiong, D.; Ye, Y.L.; Fu, Y.J.; Wang, J.L.; Kuang, B.H.; Wang, H.B.; Wang, X.M.; Zu, L.D.; Xiao, G.; Hao, M.G.; et al. Bmi-1 expression modulated non-small cell lung cancer progression. Cancer Biol. Ther. 2015, 16, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Hagen, N.; Gao, L.; Tkaczyk, T.S. Quantitative sectioning and noise analysis for structured illumination microscopy. Opt. Express 2012, 20, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.L.; Mueller, J.L.; Javid, M.P.; Mito, J.K.; Kirsch, D.G.; Ramanujam, N.; Brown, J.Q. Optimization of a Widefield Structured Illumination Microscope for Non-Destructive Assessment and Quantification of Nuclear Features in Tumor Margins of a Primary Mouse Model of Sarcoma. PLoS ONE 2013, 8, e68868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolg, M.; Stoll, H.; Savin, A.; Preuss, H. Energy-adjusted pseudopotentials for the rare earth elements. Theor. Chim. Acta 1989, 75, 173–194. [Google Scholar] [CrossRef]
- Dolg, M.; Stoll, H.; Preuss, H. A combination of quasirelativistic pseudopotential and ligand field calculations for lanthanoid compounds. Theor. Chim. Acta 1993, 85, 441–450. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis Set Exchange: A Community Database for Computational Sciences. J. Chem. Inf. Model 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pietro, S.; Iacopini, D.; Storti, B.; Nifosì, R.; Di Bussolo, V.; Pineschi, M.; Moscardini, A.; Signore, G.; Bizzarri, R. New 1,3-Disubstituted Benzo[h]Isoquinoline Cyclen-Based Ligand Platform: Synthesis, Eu3+ Multiphoton Sensitization and Imaging Applications. Molecules 2021, 26, 58. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010058
Di Pietro S, Iacopini D, Storti B, Nifosì R, Di Bussolo V, Pineschi M, Moscardini A, Signore G, Bizzarri R. New 1,3-Disubstituted Benzo[h]Isoquinoline Cyclen-Based Ligand Platform: Synthesis, Eu3+ Multiphoton Sensitization and Imaging Applications. Molecules. 2021; 26(1):58. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010058
Chicago/Turabian StyleDi Pietro, Sebastiano, Dalila Iacopini, Barbara Storti, Riccardo Nifosì, Valeria Di Bussolo, Mauro Pineschi, Aldo Moscardini, Giovanni Signore, and Ranieri Bizzarri. 2021. "New 1,3-Disubstituted Benzo[h]Isoquinoline Cyclen-Based Ligand Platform: Synthesis, Eu3+ Multiphoton Sensitization and Imaging Applications" Molecules 26, no. 1: 58. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26010058