The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of H2L and the Manganese(II), Cobalt(II) and Zinc(II) Complexes
2.2. NMR Studies
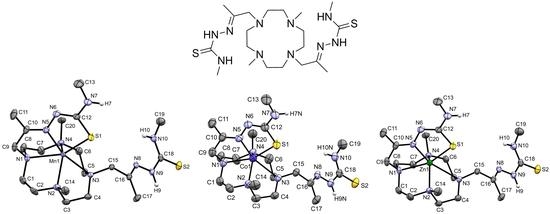
2.3. X-ray Crystal Structures of [MnHL](BPh4), [CoHL](BPh4) and [ZnHL](BPh4)
2.4. Magnetic Susceptibility
2.5. Density Functional Theory Calculations
2.6. Radiolabelling with 68Ga3+
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
General Procedure for the Synthesis of the Complexes
3.3. Radiolabelling with 68Ga
3.4. Single-Crystal X-ray Diffraction Procedure
3.5. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Richardson, D.; Kalinowski, D.S.; Richardson, V.; Sharpe, P.; Lovejoy, D.B.; Islam, M.; Bernhardt, P.V. 2-Acetylpyridine Thiosemicarbazones are Potent Iron Chelators and Antiproliferative Agents: Redox Activity, Iron Complexation and Characterization of their Antitumor Activity. J. Med. Chem. 2009, 52, 1459–1470. [Google Scholar] [CrossRef]
- Pedrido, R.; González-Noya, A.M.; Romero, M.J.; Martínez-Calvo, M.; López, M.V.; Gómez-Fórneas, E.; Zaragoza, G.; Bermejo, M.R.; Verez, G.Z. Pentadentate thiosemicarbazones as versatile chelating systems. A comparative structural study of their metallic complexes. Dalton Trans. 2008, 6776–6787. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, K.; Sekino, K.; Ishikawa, M.; Honda, A.; Yokoyama, M.; Kasuga, N.C.; Yokoyama, H.; Nakano, S.; Onodera, K. Syntheses, crystal structures and antimicrobial activities of monomeric 8-coordinate, and dimeric and monomeric 7-coordinate bismuth(III) complexes with tridentate and pentadentate thiosemicarbazones and pentadentate semicarbazone ligands. J. Inorg. Biochem. 2004, 98, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Paterson, B.M.; White, J.M.; Donnelly, P.S. A hexadentate bis(thiosemicarbazonato) ligand: Rhenium(V), iron(III) and cobalt(III) complexes. Dalton Trans. 2010, 39, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Pavlishchuk, V.V.; Kolotilov, S.V.; Addison, A.W.; Butcher, R.J.; Sinn, E. Nickel(II) complexes with dithiadiiminoamine and dithiabis(thiosemicarbazone) ligands. J. Chem. Soc. Dalton Trans. 2000, 335–341. [Google Scholar] [CrossRef]
- Zaltariov, M.F.; Hammerstad, M.; Arabshahi, H.J.; Jovanović, K.; Richter, K.W.; Cazacu, M.; Shova, S.; Balan, M.; Andersen, N.H.; Radulović, S.; et al. New Iminodiacetate–Thiosemicarbazone Hybrids and Their Copper(II) Complexes Are Potential Ribonucleotide Reductase R2 Inhibitors with High Antiproliferative Activity. Inorg. Chem. 2017, 56, 3532–3549. [Google Scholar] [CrossRef] [Green Version]
- Paterson, B.M.; White, K.F.; White, J.M.; Abrahams, B.F.; Donnelly, P.S. Guest-induced Assembly of Bis(thiosemicarbazonato) Zinc(II) Coordination Nanotubes. Angew. Chem. Int. Ed. 2017, 56, 8370–8374. [Google Scholar] [CrossRef]
- Dayal, D.; Palanimuthu, D.; Shinde, S.V.; Somasundaram, K.; Samuelson, A.G. A novel zinc bis(thiosemicarbazone) complex for live cell imaging. JBIC J. Biol. Inorg. Chem. 2011, 16, 621–632. [Google Scholar] [CrossRef]
- Huseynova, M.; Medjidov, A.; Taslimi, P.; Aliyeva, M. Synthesis, characterization, crystal structure of the coordination polymer Zn(II) with thiosemicarbazone of glyoxalic acid and their inhibitory properties against some metabolic enzymes. Bioorg. Chem. 2019, 83, 55–62. [Google Scholar] [CrossRef]
- Crouch, P.J.; Hung, L.W.; Adlard, P.A.; Cortes, M.; Lal, V.; Filiz, G.; Perez, K.A.; Nurjono, M.; Caragounis, A.; Du, T.; et al. Increasing Cu bioavailability inhibits A oligomers and tau phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Maia, P.I.D.S.; Souza, P.C.; Pavan, F.R.; Leite, C.Q.F.; Viana, R.B.; Batista, A.A.; Nascimento, O.R.; Deflon, V.M. Manganese(II) complexes with thiosemicarbazones as potential anti-Mycobacterium tuberculosis agents. J. Inorg. Biochem. 2014, 132, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, H.; Gambino, D. The Wide Pharmacological Versatility of Semicarbazones, Thiosemicarbazones and Their Metal Complexes. Mini-Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar] [CrossRef]
- Paterson, B.; Donnelly, P.S. Copper complexes of bis(thiosemicarbazones): From chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem. Soc. Rev. 2011, 40, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, J.R.; Hueting, R. Metal complexes of thiosemicarbazones for imaging and therapy. Inorg. Chim. Acta 2012, 389, 3–15. [Google Scholar] [CrossRef]
- Mendes, I.C.; Soares, M.A.; Dos Santos, R.G.; Pinheiro, C.; Beraldo, H. Gallium(III) complexes of 2-pyridineformamide thiosemicarbazones: Cytotoxic activity against malignant glioblastoma. Eur. J. Med. Chem. 2009, 44, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Chen, C.L.; Zhang, D.; Niu, J.Y.; Ji, B.S. Mn(II), Co(II) and Zn(II) complexes with heterocyclic substituted thiosemicarbazones: Synthesis, characterization, X-ray crystal structures and antitumor comparison. Eur. J. Med. Chem. 2010, 45, 3169–3177. [Google Scholar] [CrossRef]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the Old to New: Iron Chelators That Are More Than Just Ribonucleotide Reductase Inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [Google Scholar] [CrossRef]
- Li, M.-X.; Zhang, D.; Zhang, L.-Z.; Niu, J.-Y.; Ji, B.-S. Synthesis, crystal structures and biological activities of 2-acetylpyridine N(4)-cyclohexylthiosemicarbazone and its manganese(II) and nickel(II) complexes. Inorg. Chem. Commun. 2010, 13, 1572–1575. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Sharpe, P.C.; Islam, M.; Lovejoy, D.B.; Kalinowski, D.S.; Richardson, D.R. Iron Chelators of the Dipyridylketone Thiosemicarbazone Class: Precomplexation and Transmetalation Effects on Anticancer Activity. J. Med. Chem. 2009, 52, 407–415. [Google Scholar] [CrossRef]
- Stacy, A.E.; Palanimuthu, D.; Bernhardt, P.V.; Kalinowski, D.S.; Jansson, P.J.; Richardson, D.R. Zinc(II)–Thiosemicarbazone Complexes Are Localized to the Lysosomal Compartment Where They Transmetallate with Copper Ions to Induce Cytotoxicity. J. Med. Chem. 2016, 59, 4965–4984. [Google Scholar] [CrossRef]
- Arion, V.B.; Jakupec, M.; Galanski, M.; Unfried, P.; Keppler, B.K. Synthesis, structure, spectroscopic and in vitro antitumour studies of a novel gallium(III) complex with 2-acetylpyridine 4N-dimethylthiosemicarbazone. J. Inorg. Biochem. 2002, 91, 298–305. [Google Scholar] [CrossRef]
- Kowol, C.R.; Berger, R.; Eichinger, R.; Roller, A.; Jakupec, M.; Schmidt, P.P.; Arion, V.B.; Keppler, B.K. Gallium(III) and Iron(III) Complexes of α-N-Heterocyclic Thiosemicarbazones: Synthesis, Characterization, Cytotoxicity, and Interaction with Ribonucleotide Reductase. J. Med. Chem. 2007, 50, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Deng, J.; Qian, K.; Tian, L.; Li, J.; He, K.; Huang, X.; Cheng, Z.; Zheng, Y.; Wang, Y. Novel 2-pyridinecarboxaldehyde thiosemicarbazones Ga(III) complexes with a high antiproliferative activity by promoting apoptosis and inhibiting cell cycle. Eur. J. Med. Chem. 2017, 134, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Qian, K.; Tian, L.; Cheng, Z.; Wang, Y. Gallium(III)–2-benzoylpyridine-thiosemicarbazone complexes promote apoptosis through Ca2+ signaling and ROS-mediated mitochondrial pathways. New J. Chem. 2018, 42, 10226–10233. [Google Scholar] [CrossRef]
- King, A.P.; Gellineau, H.A.; Ahn, J.-E.; MacMillan, S.N.; Wilson, J.J. Bis(thiosemicarbazone) Complexes of Cobalt(III). Synthesis, Characterization, and Anticancer Potential. Inorg. Chem. 2017, 56, 6609–6623. [Google Scholar] [CrossRef]
- West, D.X.; Nassar, A.; El-Saied, F.A.; Ayad, M.I. Cobalt(II) complexes with 2-aminoacetophenone N(4)-substituted thiosemicarbazones. Transit. Met. Chem. 1999, 24, 617–621. [Google Scholar] [CrossRef]
- Deng, J.; Li, T.; Su, G.; Qin, Q.-P.; Liu, Y.; Gou, Y. Co(III) complexes based on α-N-heterocyclic thiosemicarbazone ligands: DNA binding, DNA cleavage, and topoisomerase I/II inhibitory activity studies. J. Mol. Struct. 2018, 1167, 33–43. [Google Scholar] [CrossRef]
- Di Vaira, M.; Bazzicalupi, C.; Orioli, P.; Messori, L.; Bruni, A.B.; Zatta, P. Clioquinol, a Drug for Alzheimer’s Disease Specifically Interfering with Brain Metal Metabolism: Structural Characterization of Its Zinc(II) and Copper(II) Complexes. Inorg. Chem. 2004, 43, 3795–3797. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Yasui, H. Zinc complexes developed as metallopharmaceutics for treating diabetes mellitus based on the bio-medicinal inorganic chemistry. Curr. Top. Med. Chem. 2012, 12, 210–218. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Mashele, S.S.; Eze, K.C.; Matowane, G.R.; Islam, S.M.; Bonnet, S.L.; Noreljaleel, A.E.; Ramorobi, L.M. A comprehensive review on zinc(II) complexes as anti-diabetic agents: The advances, scientific gaps and prospects. Pharmacol. Res. 2020, 155, 104744. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wei, J.-H.; Chen, Z.-F.; Liu, M.; Gu, Y.-Q.; Huang, K.-B.; Li, Z.-Q.; Liang, H. The antitumor activity of zinc(II) and copper(II) complexes with 5,7-dihalo-substituted-8-quinolinoline. Eur. J. Med. Chem. 2013, 69, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, J.; Khan, Z.H.; Ma, F.; Liu, X. A novel zinc complex with antibacterial and antioxidant activity. BMC Chem. 2021, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, N.C.; Sekino, K.; Ishikawa, M.; Honda, A.; Yokoyama, M.; Nakano, S.; Shimada, N.; Koumo, C.; Nomiya, K. Synthesis, structural characterization and antimicrobial activities of 12 zinc(II) complexes with four thiosemicarbazone and two semicarbazone ligands. J. Inorg. Biochem. 2003, 96, 298–310. [Google Scholar] [CrossRef]
- Tarushi, A.; Totta, X.; Raptopoulou, C.; Psycharis, V.; Psomas, G.; Kessissoglou, D.P. Structural features of mono- and tri-nuclear Zn(II) complexes with a non-steroidal anti-inflammatory drug as ligand. Dalton Trans. 2012, 41, 7082. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H.; Konishi, J.; Yokoyama, A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38, 1155–1160. [Google Scholar]
- Torres, J.B.; Andreozzi, E.M.; Dunn, J.T.; Siddique, M.; Szanda, I.; Howlett, D.R.; Sunassee, K.; Blowera, P.J.; Torres, J.B. PET Imaging of Copper Trafficking in a Mouse Model of Alzheimer Disease. J. Nucl. Med. 2015, 57, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Andreozzi, E.M.; Torres, J.B.; Sunassee, K.; Dunn, J.; Walker-Samuel, S.; Szanda, I.; Blower, P.J. Studies of copper trafficking in a mouse model of Alzheimer’s disease by positron emission tomography: Comparison of 64Cu acetate and 64CuGTSM. Metallomics 2017, 9, 1622–1633. [Google Scholar] [CrossRef] [Green Version]
- Paterson, B.M.; Cullinane, C.; Crouch, P.J.; White, A.R.; Barnham, K.J.; Roselt, P.D.; Noonan, W.; Binns, D.; Hicks, R.J.; Donnelly, P.S. Modification of Biodistribution and Brain Uptake of Copper Bis(thiosemicarbazonato) Complexes by the Incorporation of Amine and Polyamine Functional Groups. Inorg. Chem. 2019, 58, 4540–4552. [Google Scholar] [CrossRef]
- Hickey, J.L.; Lim, S.; Hayne, D.; Paterson, B.; White, J.M.; Villemagne, V.L.; Roselt, P.; Binns, D.; Cullinane, C.; Jeffery, C.M.; et al. Diagnostic Imaging Agents for Alzheimer’s Disease: Copper Radiopharmaceuticals that Target Aβ Plaques. J. Am. Chem. Soc. 2013, 135, 16120–16132. [Google Scholar] [CrossRef]
- McInnes, L.E.; Noor, A.; Kysenius, K.; Cullinane, C.; Roselt, P.; McLean, C.A.; Chiu, F.C.K.; Powell, A.K.; Crouch, P.J.; White, J.M.; et al. Potential Diagnostic Imaging of Alzheimer’s Disease with Copper-64 Complexes That Bind to Amyloid-β Plaques. Inorg. Chem. 2019, 58, 3382–3395. [Google Scholar] [CrossRef]
- Cowley, A.R.; Dilworth, J.R.; Donnelly, P.S.; Heslop, J.M.; Ratcliffe, S.J. Bifunctional chelators for copper radiopharmaceuticals: The synthesis of [Cu(ATSM)–amino acid] and [Cu(ATSM)–octreotide] conjugates. Dalton Trans. 2007, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Paterson, B.; Karas, J.A.; Scanlon, D.B.; White, J.M.; Donnelly, P.S. Versatile New Bis(thiosemicarbazone) Bifunctional Chelators: Synthesis, Conjugation to Bombesin(7−14)-NH2, and Copper-64 Radiolabeling. Inorg. Chem. 2010, 49, 1884–1893. [Google Scholar] [CrossRef]
- Hueting, R.; Christlieb, M.; Dilworth, J.R.; Garayoa, E.G.; Gouverneur, V.; Jones, M.W.; Maes, V.; Schibli, R.; Sun, X.; Tourwé, D.A. Bis(thiosemicarbazones) as bifunctional chelators for the room temperature 64-copper labeling of peptides. Dalton Trans. 2010, 39, 3620–3632. [Google Scholar] [CrossRef] [PubMed]
- Rösch, F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013, 76, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schafer, M.A.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef]
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A.; Munteanu, C.R.; Cakebread, A.; Imberti, C.; Eykyn, T.R.; Young, J.D.; Paterson, B.M.; Blower, P.J.; et al. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinnes, J.-P.; Nagel, J.; Waldron, B.P.; Maina, T.; Nock, B.A.; Bergmann, R.K.; Ullrich, M.; Pietzsch, J.; Bachmann, M.; Baum, R.P.; et al. Instant kit preparation of 68Ga-radiopharmaceuticals via the hybrid chelator DATA: Clinical translation of [68Ga]Ga-DATA-TOC. EJNMMI Res. 2019, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Joshi, T.; Graham, B.; Spiccia, L. Macrocyclic Metal Complexes for Metalloenzyme Mimicry and Sensor Development. Accounts Chem. Res. 2015, 48, 2366–2379. [Google Scholar] [CrossRef]
- Lejault, P.; Duskova, K.; Bernhard, C.; Valverde, I.E.; Romieu, A.; Monchaud, D. The Scope of Application of Macrocyclic Polyamines Beyond Metal Chelation. Eur. J. Org. Chem. 2019, 2019, 6146–6157. [Google Scholar] [CrossRef]
- Shinoda, S. Dynamic cyclen–metal complexes for molecular sensing and chirality signaling. Chem. Soc. Rev. 2013, 42, 1825–1835. [Google Scholar] [CrossRef] [Green Version]
- Rashid, H.U.; Martines, M.A.U.; Jorge, J.; De Moraes, P.M.; Umar, M.N.; Khan, K.; Rehman, H.U. Cyclen-based Gd3+ complexes as MRI contrast agents: Relaxivity enhancement and ligand design. Bioorg. Med. Chem. 2016, 24, 5663–5684. [Google Scholar] [CrossRef] [PubMed]
- Cabbiness, D.K.; Margerum, D.W. Macrocyclic effect on the stability of copper(II) tetramine complexes. J. Am. Chem. Soc. 1969, 91, 6540–6541. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. The Chelate, Cryptate and Macrocyclic Effects. Comments Inorg. Chem. 1988, 6, 237–284. [Google Scholar] [CrossRef]
- Baranyai, Z.; Tircsó, G.; Rösch, F. The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations. Eur. J. Inorg. Chem. 2020, 2020, 36–56. [Google Scholar] [CrossRef] [Green Version]
- Stasiuk, G.; Long, N.J. The ubiquitous DOTA and its derivatives: The impact of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid on biomedical imaging. Chem. Commun. 2013, 49, 2732–2746. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.P.; Clemente, G.S.; Archibald, S.J. Recent advances in chelator design and labelling methodology for 68Ga radiopharmaceuticals. J. Label. Compd. Radiopharm. 2014, 57, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Molnár, E.; Camus, N.; Patinec, V.; Rolla, G.A.; Botta, M.; Tircsó, G.; Kálmán, F.K.; Fodor, T.; Tripier, R.; Platas-Iglesias, C. Picolinate-Containing Macrocyclic Mn2+ Complexes as Potential MRI Contrast Agents. Inorg. Chem. 2014, 53, 5136–5149. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Knigge, U.; Mortensen, J.; Oturai, P.; Berthelsen, A.K.; Loft, A.; Binderup, T.; Rasmussen, P.; Elema, D.; Klausen, T.L.; et al. Clinical PET of Neuroendocrine Tumors Using 64Cu-DOTATATE: First-in-Humans Study. J. Nucl. Med. 2012, 53, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Demirci, E.; Kabasakal, L.; Toklu, T.; Ocak, M.; Şahin, O.E.; Alan-Selcuk, N.; Araman, A. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours including high-grade (WHO G3) neuroendocrine tumours: Response to treatment and long-term survival update. Nucl. Med. Commun. 2018, 39, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.L.; Davey, P.R.W.J.; Ma, M.T.; White, J.M.; Morgenstern, A.; Bruchertseifer, F.; Blower, P.J.; Paterson, B.M. An octadentate bis(semicarbazone) macrocycle: A potential chelator for lead and bismuth radiopharmaceuticals. Dalton Trans. 2020, 49, 14962–14974. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.S.; Arrowsmith, R.L.; Cortezon-Tamarit, F.; Twyman, F.; Kociok-Köhn, G.; Botchway, S.W.; Dilworth, J.R.; Carroll, L.; Aboagye, E.O.; Pascu, S.I. Microwave gallium-68 radiochemistry for kinetically stable bis(thiosemicarbazone) complexes: Structural investigations and cellular uptake under hypoxia. Dalton Trans. 2015, 45, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Arrowsmith, R.L.; Waghorn, P.A.; Jones, M.W.; Bauman, A.; Brayshaw, S.K.; Hu, Z.; Kociok-Kohn, G.D.; Mindt, T.L.; Tyrrell, R.M.; Botchway, S.W.; et al. Fluorescent gallium and indium bis(thiosemicarbazonates) and their radiolabelled analogues: Synthesis, structures and cellular confocal fluorescence imaging investigations. Dalton Trans. 2011, 40, 6238–6252. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Mehdipour, P.; Akhlaghi, M.; Yousefnia, H.; Shafaii, K. Evaluation of a [67Ga]-Thiosemicarbazone Complex as Tumor Imaging Agent. Sci. Pharm. 2009, 77, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, F.H.; Jalilian, A.R.; Nemati, A.; Abedini, M. Preparation and biodistribution studies of [67Ga]2-acetylpyridine 4,4-dimethyl thiosemicarbazone complex as a possible SPECT tracer for detection of malignancies. J. Radioanal. Nucl. Chem. 2007, 272, 115–121. [Google Scholar] [CrossRef]
- Lima, L.; Beyler, M.; Delgado, R.; Platas-Iglesias, C.; Tripier, R. Investigating the Complexation of the Pb2+/Bi3+ Pair with Dipicolinate Cyclen Ligands. Inorg. Chem. 2015, 54, 7045–7057. [Google Scholar] [CrossRef]
- Corey, E.J.; Bailar, J.C., Jr. The Stereochemistry of Complex Inorganic Compounds. XXII. Stereospecific Effects in Complex Ions. J. Am. Chem. Soc. 1959, 81, 2620–2629. [Google Scholar] [CrossRef]
- Eisenberg, R.; Brennessel, W.W. Redetermination of the trigonal prismatic complex tris(cis-1,2-diphenylethylene-1,2-dithiolato)rhenium. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, 464–466. [Google Scholar] [CrossRef]
- Tsuboyama, S.; Matsudo, M.; Tsuboyama, K.; Sakurai, T. Structures of [(R)- and (S)-prolinato](optically active cyclen)cobalt(III) complexes. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 872–876. [Google Scholar] [CrossRef]
- Danker, F.; Näther, C.; Bensch, W. Synthesis and crystal structure of (1, 4, 7, 10-tetraazacyclododecane-κ4N)(tetrasulfido-κ2S1, S4) manganese(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Vargová, Z.; Kotek, J.; Rudovsky, J.; Plutnar, J.; Gyepes, R.; Hermann, P.; Györyová, K.; Lukeš, I. Ternary Complexes of Zinc(II), Cyclen and Pyridinecarboxylic Acids. Eur. J. Inorg. Chem. 2007, 2007, 3974–3987. [Google Scholar] [CrossRef]
- Martinelli, J.; Balali-Mood, B.; Panizzo, R.; Lythgoe, M.F.; White, A.J.P.; Ferretti, P.; Steinke, J.H.G.; Vilar, R. Coordination chemistry of amide-functionalised tetraazamacrocycles: Structural, relaxometric and cytotoxicity studies. Dalton Trans. 2010, 39, 10056–10067. [Google Scholar] [CrossRef] [Green Version]
- El Safadi, M.; Bhadbhade, M.; Shimmon, R.; Baker, A.T.; McDonagh, A.M. Cyclen-based chelators for the inhibition of Aβ aggregation: Synthesis, anti-oxidant and aggregation evaluation. Inorg. Chim. Acta 2017, 467, 343–350. [Google Scholar] [CrossRef]
- Bernier, N.; Costa, J.; Delgado, R.; Félix, V.; Royal, G.; Tripier, R. trans-Methylpyridine cyclen versus cross-bridged trans-methylpyridine cyclen. Synthesis, acid–base and metal complexation studies (metal = Co2+, Cu2+, and Zn2+). Dalton Trans. 2011, 40, 4514–4526. [Google Scholar] [CrossRef]
- Tsitovich, P.B.; Tittiris, T.Y.; Cox, J.M.; Benedict, J.B.; Morrow, J.R. Fe(II) and Co(II) N-methylated CYCLEN complexes as paraSHIFT agents with large temperature dependent shifts. Dalton Trans. 2017, 47, 916–924. [Google Scholar] [CrossRef]
- Knight, J.C.; Alvarez, S.; Angelo, J.A.; Edwards, P.G.; Singh, N. A novel bipyridine-based hexadentate tripodal framework with a strong preference for trigonal prismatic co-ordination geometries. Dalton Trans. 2010, 39, 3870–3883. [Google Scholar] [CrossRef]
- Aoki, S.; Zulkefeli, M.; Shiro, M.; Kimura, E. New supramolecular trigonal prisms from zinc(II)-1,4,7,10-tetraazacyclododecane (cyclen) complexes and trithiocyanurate in aqueous solution. Proc. Natl. Acad. Sci. USA 2002, 99, 4894–4899. [Google Scholar] [CrossRef] [Green Version]
- Kojima, M.; Nakabayashi, K.; Ohba, S.; Okumoto, S.; Saito, Y.; Fujita, J. Green and Brown Isomers of the ((S)-1-Amino-2-propanethiolato-N,S)(1,4,7,10-tetraazacyclododecane)cobalt(III) Ion and Crystal Structure of the Green Isomer. Bull. Chem. Soc. Jpn. 1986, 59, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S. and Sangeetika. Synthesis and spectral studies on copper(II) and cobalt(II) complexes of macrocyclic ligand containing thiosemicarbazone moiety. Ind. J. Chem. A 2002, 41, 1629. [Google Scholar]
- Chandra, S.; Pundir, M. Spectral studies of cobalt(II) complexes of 12-membered macrocyclic ligands having thiosemicarbazone moieties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Tsitovich, P.B.; Morrow, J.R. Macrocyclic ligands for Fe(II) paraCEST and chemical shift MRI contrast agents. Inorg. Chim. Acta 2012, 393, 3–11. [Google Scholar] [CrossRef]
- Howard, J.A.K.; Kenwright, A.; Moloney, J.M.; Parker, D.; Woods, M.; Port, M.; Navet, M.; Rousseau, O. Structure and dynamics of all of the stereoisomers of europium complexes of tetra(carboxyethyl) derivatives of dota: Ring inversion is decoupled from cooperative arm rotation in the RRRR and RRRS isomers. Chem. Commun. 1998, 1381–1382. [Google Scholar] [CrossRef]
- Enamullah, M.; Vasylyeva, V.; Janiak, C. Chirality and diastereoselection of Δ/Λ-configured tetrahedral zinc(II) complexes with enantiopure or racemic Schiff base ligands. Inorg. Chim. Acta 2013, 408, 109–119. [Google Scholar] [CrossRef]
- Chamayou, A.-C.; Lüdeke, S.; Brecht, V.; Freedman, T.B.; Nafie, L.A.; Janiak, C. Chirality and Diastereoselection of Δ/Λ-Configured Tetrahedral Zinc Complexes through Enantiopure Schiff Base Complexes: Combined Vibrational Circular Dichroism, Density Functional Theory, 1H NMR, and X-ray Structural Studies. Inorg. Chem. 2011, 50, 11363–11374. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Lüdeke, S.; Chamayou, A.-C.; Marolt, M.; Justus, V.; Górecki, M.; Arrico, L.; Di Bari, L.; Islam, M.A.; Gruber, I.; et al. Broad-Range Spectral Analysis for Chiral Metal Coordination Compounds: (Chiro)optical Superspectrum of Cobalt(II) Complexes. Inorg. Chem. 2018, 57, 13397–13408. [Google Scholar] [CrossRef]
- Vargas, A.; Zerara, M.; Krausz, E.; Hauser, A.; Daku, L.M.L. Density-Functional Theory Investigation of the Geometric, Energetic, and Optical Properties of the Cobalt(II)tris(2,2′-bipyridine) Complex in the High-Spin and the Jahn−Teller Active Low-Spin States. J. Chem. Theory Comput. 2006, 2, 1342–1359. [Google Scholar] [CrossRef] [Green Version]
- Wadas, T.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef] [Green Version]
- Prado, V.D.S.; Leitao, R.C.F.; Silva, F.; Gano, L.; Santos, I.C.; Marques, F.L.N.; Paulo, A.R.; Deflon, V.M. Gallium and indium complexes with new hexadentate bis(semicarbazone) and bis(thiosemicarbazone) chelators. Dalton Trans. 2021, 50, 1631–1640. [Google Scholar] [CrossRef]
- Earnshaw, A. Introduction to Magnetochemistry; Elsevier: London, UK, 1968. [Google Scholar]
- Kahn, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993. [Google Scholar]
- CrysAlisPro; Rigaku Oxford Diffraction: Yarnton, UK, 2018.
- Sheldrick, G. Crystal Structure refinement with SHELXL. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Dennington, T.A.K.R.; Millam, J.M. GaussView; Version 6.1.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]







| [MnHL](BPh4) | [CoHL](BPh4)·(C3H6O) | [ZnHL](BPh4)·1.67(C3H6O) | |
|---|---|---|---|
| Empirical formula | C44H61BMnN10S2 | C47H67BCoN10OS2 | C49H70.75BN10O1.67S2Zn |
| Formula weight | 859.89 | 921.96 | 966.86 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P21/c |
| Temperature (K) | 123(2) | 173(2) | 123(1) |
| a (Å) | 17.4842(3) | 17.2017(2) | 17.32220(10) |
| b (Å) | 35.9904(5) | 35.8919(4) | 36.0585(2) |
| c (Å) | 17.4662(3) | 17.3716(2) | 17.36950(10) |
| α (°) | 90 | 90 | 90 |
| β (°) | 100.8860(10) | 102.2170(10) | 101.7190(10) |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 10,793.1(3) | 10,482.4 | 10,623.06(11) |
| Z | 8 | 8 | 8 |
| Dc (g cm−3) | 1.058 | 1.168 | 1.209 |
| Absorption coefficient (mm−1) | 0.358 | 3.639 | 1.729 |
| F(000) | 3656 | 3928 | 4121 |
| Angle range 2θ, ° | MoKα 6.630 to 55.754 | CuKα 7.168 to 154.048 | CuKα 7.012 to 154.846 |
| Reflections collected | 143,110 | 102,026 | 115,251 |
| Independent reflections | 25,679 [R(int) = 0.0469] | 21,504 [R(int) = 0.0832] | 22,186 [R(int) = 0.0456] |
| Final R1 values (I > 2σ(I)) | 0.0440 | 0.0889 | 0.0572 |
| Final wR1(F2) values (I > 2σ(I)) | 0.1111 | 0.2389 | 0.1622 |
| Final R1 values (all data) | 0.0606 | 0.1252 | 0.0617 |
| Final wR1(F2) values (all data) | 0.1180 | 0.2764 | 0.1667 |
| GoF on F2 | 1.076 | 1.036 | 1.023 |
| CSD no. | 2,072,659 | 2,072,660 | 2,072,661 |
| Mn1 (Å) | Mn2 (Å) | Co1 (Å) | Co2 (Å) | Zn1 (Å) | Zn2 (Å) | |
|---|---|---|---|---|---|---|
| M-N1/11 | 2.362(2) | 2.397(1) | 2.331(4) | 2.325(4) | 2.340(2) | 2.393(2) |
| M-N2/12 | 2.264(2) | 2.254(2) | 2.156(4) | 2.183(5) | 2.152(2) | 2.140(2) |
| M-N3/13 | 2.501(2) | 2.471(2) | 2.620(4) | 2.317(4) | 2.737(2) | 2.630(2) |
| M-N4/14 | 2.244(2) | 2.250(2) | 2.113(4) | 2.159(4) | 2.121(2) | 2.125(2) |
| M-N5/15 | 2.257(2) | 2.218(2) | 2.120(4) | 2.087(4) | 2.147(2) | 2.130(2) |
| M-S1/3 | 2.462(1) | 2.455(1) | 2.323(2) | 2.365(1) | 2.346(7) | 2.344(7) |
| θ1 (°) | θ2 (°) | θ3 (°) | Av (°) | |
|---|---|---|---|---|
| Mn1 | 35.7 | 20.2 | 8.1 | 21.4 |
| Mn2 | 32.0 | 18.6 | 7.0 | 19.2 |
| Co1 | 37.5 | 21.4 | 12.3 | 23.7 |
| Co2 | 40.7 | 31.2 | 20.9 | 30.9 |
| Zn1 | 37.4 | 21.7 | 13.1 | 24.1 |
| Zn2 | 33.7 | 21.5 | 12.6 | 22.6 |
| Zn1 XRD | Zn1 DFT | Zn2 XRD | Zn2 DFT | Co1 XRD | Co1 DFT | Co2 XRD | Co2 DFT | |
|---|---|---|---|---|---|---|---|---|
| M-N1/N11 | 2.340(2) | 2.390 | 2.393(2) | 2.411 | 2.331(4) | 2.351 | 2.325(4) | 2.402 |
| M-N2/N12 | 2.151(2) | 2.240 | 2.140(2) | 2.260 | 2.156(4) | 2.248 | 2.183(5) | 2.259 |
| M-N3/N13 | 2.737(2) | 2.727 | 2.630(2) | 2.701 | 2.620(4) | 2.680 | 2.317(4) | 2.400 |
| M-N4/N14 | 2.120(2) | 2.228 | 2.125(2) | 2.238 | 2.113(4) | 2.217 | 2.159(4) | 2.239 |
| M-N5N/15 | 2.147(2) | 2.211 | 2.130(2) | 2.212 | 2.120(4) | 2.164 | 2.087(4) | 2.128 |
| M-S1/S3 | 2.346(7) | 2.420 | 2.344(7) | 2.419 | 2.323(2) | 2.401 | 2.365(1) | 2.412 |
| H2L | DOTA | |||
|---|---|---|---|---|
| pH 3.5 | pH 6 | pH 3.5 | pH 6 | |
| 25 °C | 1.8 ± 1.7 | 19.8 ± 2.6 | 86.7 ± 5.0 | 73.2 ± 6.4 |
| 40 °C | 60.4 ± 3.1 | 70.3 ± 1.9 | - | - |
| 90 °C | 95.1 ± 2.3 | 89.0 ± 4.3 | 95.3 ± 0.9 | 97.2 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieve, M.L.; Davey, P.R.W.J.; Forsyth, C.M.; Paterson, B.M. The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes. Molecules 2021, 26, 3646. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123646
Grieve ML, Davey PRWJ, Forsyth CM, Paterson BM. The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes. Molecules. 2021; 26(12):3646. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123646
Chicago/Turabian StyleGrieve, Melyssa L., Patrick R. W. J. Davey, Craig M. Forsyth, and Brett M. Paterson. 2021. "The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes" Molecules 26, no. 12: 3646. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26123646