Assessment of the Botanical Origin of Polish Honeys Based on Physicochemical Properties and Bioactive Components with Chemometric Analysis
Abstract
:1. Introduction
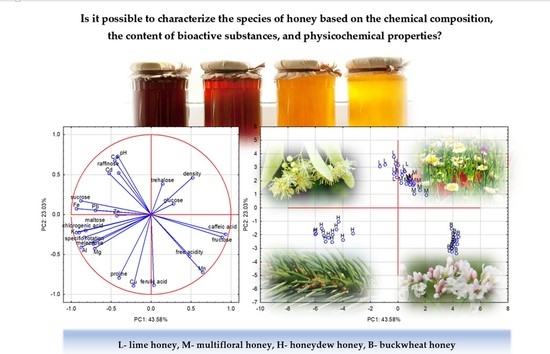
2. Results and Discussion
3. Chemometric Analysis
4. Materials and Methods
4.1. Honey Samples
4.2. Analytical Procedures
4.2.1. Profiles of Phenolic Compounds
4.2.2. Proline Content
4.2.3. Mineral Content
4.2.4. Sugar Profile
4.2.5. Physicochemical Properties
4.3. Statistical Analysis
4.4. Chemicals and Reagents
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Puścion-Jakubik, A.; Borawska, M.H.; Socha, K. Modern Methods for Assessing the Quality of Bee Honey and Botanical Origin Identification. Foods 2020, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Nayik, G.A.; Nanada, V. Physico-Chemical, Enzymatic, Mineral and Colour Characterization of Three Different Varieties of Honeys from Kashmir Valley of India with a Multivariate Approach. Pol. J. Food Nutr. Sci. 2015, 65, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Machado De-Melo, A.A.; De Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Mate’, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2017, 57, 5–37. [Google Scholar] [CrossRef]
- Oroian, M.; Sorina, R. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Amaral, J.S.; Oliviera, M.B.P.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, A.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the Botanical Origin of Honeys with Chemometric Analysis According to Their Antioxidant and Physicochemical Properties. Pol. J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Nayik, N.; Nanda, V. A chemometric approach to evaluate the phenolic compounds, antioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT-Food Sci. Technol. 2016, 74, 504–513. [Google Scholar] [CrossRef]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 145, 404–408. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Zhang, J.; Li, Y.; Chen, L.; Zhao, W.; Zhou, J.; Jin, Y. Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis. Molecules 2017, 22, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merken, H.M.; Beecher, G.R. Measurement of food flavonoids by high- performance liquid chromatography: A review. J. Agric. Food Chem. 2000, 48, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Kędzia, B.; Hołderna-Kędzia, E. Presence of phenolic compounds in bee honey. Borgis—Postęp. Fitoter. 2008, 4, 225–232. [Google Scholar]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef]
- Janiszewska, K.; Aniołowska, A.; Nowakowski, P. Free Amino Acids Content of Honeys from Poland. Pol. J. Food Nutr. Sci. 2012, 62, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, S.; Kopuncová, M.; Ciesarová, Z.; Kukurová, K. Free amino acids profile of Polish and Slovak honeys based on LC– MS/MS method without the prior derivatisation. J. Food Sci. Technol. 2017, 54, 3716–3723. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.T.; De Lorenzo, C.; Del Carmen-Polo, M.; Martin-Alvarez, P.J.; Pueyo, E. Usefulness of amino acid composition to discriminate between honeydew and floral honeys. Application to honeys from a small geographic area. J. Agric. Food Chem. 2004, 52, 84–89. [Google Scholar] [CrossRef]
- Oroian, M.; Amariei, S.; Rosu, A.; Gutt, G. Classification of unifloral honeys using multivariate analysis. J. Essent. Oil Res. 2015, 27, 533–544. [Google Scholar] [CrossRef]
- Popek, S.; Halagarda, M.; Kursa, K. A new model to identify botanical origin of Polish honeys based on the physicochemical parameters and chemometric analysis. LWT-Food Sci. Technol. 2017, 77, 482–487. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Tezcan, F.; Erima, F.B.; Yildiz, O.; Sahin, H.; Can, Z.; Kolayli, S. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT-Food Sci. Technol. 2016, 68, 273–279. [Google Scholar] [CrossRef]
- Fernández-Torres, R.; PérezBernal, J.L.; Bello-López, M.A.; Callejón-Mochón, M.; Jiménez- Sánchez, J.C.; Guiraúm-Pérez, A. Mineral content and botanical origin of Spanish honeys. Talanta 2005, 65, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Bilandžić, N.; Gajger, I.T.; Kosanović, M.; Čalopek, B.; Sedak, M.; Kolanović, B.S.; Varenina, I.; Luburić, Ð.B.; Varga, I.; Ðokić, M. Essential and toxic element concentrations in monofloral honeys from southern Croatia. Food Chem. 2017, 234, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Metals in honeys from different areas of southern Italy. Bull. Environ. Contam. Toxicol. 2014, 92, 253–258. [Google Scholar] [CrossRef]
- Czipa, N.; Andrási, D.; Kovács, B. Determination of essential and toxic elements in Hungarian honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Videira, R.; Monteiro, A.P.; Valentão, P.; Andrade, P.B. Honey from Luso region (Portugal): Physicochemical characteristics and mineral contents. Microchem. J. 2009, 93, 73–77. [Google Scholar] [CrossRef]
- Yucel, Y.; Sultanoglu, P. Characterization of Hatay honeys according to their multi-element analysis using ICP-OES combined with chemometrics. Food Chem. 2013, 140, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Chudzinska, M.; Baralkiewicz, D. Estimation of honey authenticity by multielements characteristics using inductively coupled plasma-mass spectrometry (ICP-MS) combined with chemometrics. Food Chem. Toxicol. 2010, 48, 284–290. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hicks, C.L.; Florence, R.L. Aluminum bioavailability from basic sodium aluminum phosphate, an approved food additive emulsifying agent, incorporated in cheese. Food Chem. Toxicol. 2008, 46, 2261–2266. [Google Scholar] [CrossRef] [Green Version]
- Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=298 (accessed on 15 June 2021).
- Yarsan, E.; Karacal, F.; Ibrahim, I.G.; Dikmen, B.; Koksal, A.; Das, Y.K. Contents of some metals in honeys from different regions in Turkey. Bull. Environ. Contam. Toxicol. 2007, 79, 255–258. [Google Scholar] [CrossRef]
- Silici, S.; Uluozlu, O.D.; Tuzen, M.; Soylak, M. Assessment of trace element levels in Rhododendron honeys of Black Sea Region, Turkey. J. Hazard. Mater. 2008, 156, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Vanhanen, L.P.; Emmertz, A.; Savage, G.P. Mineral analysis of mono-floral New Zealand honey. Food Chem. 2011, 128, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, P.; Wang, C.; Wu, Y. Human health risk assessment of cadmium via dietary intake by children in Jiangsu Province, China. Environ. Geochem. Health 2017, 39, 29–41. [Google Scholar] [CrossRef]
- Rostkowski, J.; Omieljaniuk, N. Determination of lead content in honey in Poland. Bromatol. Chem. Toksykol. 1989, 25, 319–327. [Google Scholar]
- Leibler, J.H.; Basra, K.; Ireland, T.; McDonagh, A.; Ressijac, C.; Heiger-Bernays, W.; Vorhees, D.; Rosenbaum, M. Lead exposure to children from consumption of backyard chicken eggs. Environ. Res. 2018, 167, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Lead Poisoning and Health—WHO. Available online: https://www.who.int/news-room/factsheets/detail/lead-poisoning-and-health (accessed on 23 August 2020).
- Pohl, P.; Stecka, H.; Greda, K.; Jamroz, P. Bioaccessibility of Ca, Cu, Fe, Mg, Mn and Zn from commercial bee honeys. Food Chem. 2012, 134, 392–396. [Google Scholar] [CrossRef]
- Zielińska, S.; Wesołowska, M.; Bilek, M.; Kaniuńczak, J.; Dżugan, M. The saccharide profile of Polish honeys depending on their botanical origin. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 387–390. [Google Scholar]
- Ojeda De Rodríguez, G.; De Ferrer, S.B.; Ferrer, A.; Rodríguez, B. Characterization of honey produced in Venezuela. Food Chem. 2004, 84, 499–502. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R.; Ceksteryte, V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. Food Sci. Technol. 2010, 43, 801–807. [Google Scholar] [CrossRef]
- Nozal, M.J.; Bernal, J.L.; Toribio, L.; Alamo, M.; Diego, J.C. The use of carbohydrate profiles and chemometrics in the characterization of natural honeys of identical geographical origin. J. Agric. Food Chem. 2005, 53, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K. Bestimmung des Zuckerspektrums in Honigen Unterschiedlicher Sorte und Herkunft mit Hilfe der HPLC. Diplomarb eit. Master’s Thesis, University of Hohenheim, Stuttgart, Germany, 2001. [Google Scholar]
- Gómez Bárez, J.A.; García-Villanova, R.J.; Elvira García, S.; Rivas Palá, T.; González Paramás, A.M.; Sánchez Sánchez, J. Geographical discrimination of honeys through the employment of sugar patterns and common chemical quality parameters. Eur. Food Res. Technol. 2000, 210, 437–444. [Google Scholar] [CrossRef]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Chromatographic analysis of sugars applied to the characterization of monofloral honey. Anal. Bioanal. Chem. 2004, 380, 698–705. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H. Porównanie Międzylaboratoryjne Wyników Badań Dotyczących Jakości Miodu, Charakterystyka Krajowych Miodów Odmianowych (Interlaboratory Comparison of Test Results on Honey Quality, Characteristics of Domestic Varietal Honeys); Instytut Ogrodnictwa: Skierniewce, Poland, 2014; pp. 1–23. [Google Scholar]
- Paranov, P.; Dinkov, D.; Tananaki, C.; Mihaylova, G. Sensorial characteristics and composition of Bulgarian’s fennel (Foeniculum vulgate Mill.) bee honey: I. Quality parameters. J. Mt. Agric. Balk. 2011, 14, 1–22. [Google Scholar]
- Persano Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, 38–81. [Google Scholar] [CrossRef]
- Waś, E.; Rybak-Chmielewska, H.; Szczęsna, T.; Kachaniuk, K.; Teper, D. Characteristics of Polish unifloral honeys. II. Lime honey (Tilia spp.). J. Apic. Sci. 2011, 55, 121–128. [Google Scholar]
- Council Directive 2014/63/EU of the European Parliament and of the Council Amending Council Directive2001/110/EC Relating to Honey. Off. J. Eur. Commun. 2014, 57, p. L164/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L:2014:164:FULL&from=EN (accessed on 15 June 2021).
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez, E.R.; Romero, C.D. Differentiation of blossom and honeydew honeys using multivariate analysis on the physicochemical parameters and sugar composition. Food Chem. 2011, 126, 664–672. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 15, 687–698. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral Markers in Honey of Various Botanical and Geographic Origins: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Dinkov, D. A scientific note on the specific optical rotation of three honey types from Bulgaria. Apidologie 2003, 34, 319–320. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, S.; Łukasiewicz, M.; Berski, W. Applicability of physico-chemical parameters of honey for identification of the botanical origin. Acta Sci. Pol. Technol. Aliment. 2013, 12, 51–59. [Google Scholar] [PubMed]
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef] [PubMed]
- Tarapatskyy, M.; Zaguła, G.; Bajcar, M.; Puchalski, C.; Saletnik, B. Magnetic Field Extraction Techniques in Preparing High-Quality Tea Infusions. Appl. Sci. 2018, 8, 1876. [Google Scholar] [CrossRef] [Green Version]
- Dżugan, M.; Zaguła, G.; Wesołowska, M.; Sowa, P.; Puchalski, C. Levels of toxic and essential metals in varietal honeys from Podkarpacie. J. Elem. 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Dżugan, M.; Sowa, P.; Kwaśniewska, M.; Wesołowska, M.; Czernicka, M. Physicochemical Parameters and Antioxidant Activity of Bee Honey Enriched With Herbs. Plant Foods Hum. Nutr. 2017, 72, 74–81. [Google Scholar] [CrossRef]
- Harmonised Methods of the International Honey Commission (IHC). Available online: http://www.bee-hexagon.net/en/network.htm (accessed on 15 April 2021).




| Component (Min.; Max.) | Multifloral | Linden | Buckwheat | Honeydew |
|---|---|---|---|---|
| Chlorogenic acid | 3.240 BGI (2.26; 3.84) | 2.040 DHa (1.46; 2.49) | 1.130 FJb (0.88; 1.43) | 6.500 ACE (5.50; 7.44) |
| Caffeic acid | 0.250 Ba (0.14; 0.34) | 0.180 Db (0.11; 0.28) | 0.520 AC (0.44; 0.62) | N/D - |
| Ferulic acid | 0.940 BbG (0.79; 1.11) | 0.530 DFH (0.43; 0.62) | 1.320 AC (1.08; 1.78) | 1.242 aE (1.10; 1.40) |
| Total identified phenolic acids | 4.434 BGI (3.28; 5.28) | 2.757 FJL (2.19; 3.35) | 2.967 DHK (2.42; 3.58) | 7.743 ACE (6.87; 8.77) |
| Proline | 184.44 FJL (172.15; 194.23) | 214.65 DHK (201.30; 228.16) | 251.57 BGI (240.33; 261.49) | 277.52 ACE (263.65; 288.65) |
| Ca | 50.080 DI (42.91; 64.36) | 83.750 ACE (82.08; 85.61) | 38.361 FHJ (35.10; 41.04) | 58.319 BG (51.27; 68.43) |
| K | 948.59 Bb (648.11; 1222.46) | 1231.95 DaG (1062.91; 1453.32) | 755.41 FH (650.23; 895.43) | 2259.39 ACE (2094.21; 2476.67) |
| Mg | 32.190 BgI (28.18; 39.29) | 24.170 DJ (20.92; 26.91) | 26.990 Fh (23.98; 31.08) | 41.960 ACE (38.01; 47.22) |
| Fe | 0.790 DH (0.52; 1.12) | 1.470 BGI (1.22; 1.68) | 0.360 FJ (0.26; 0.42) | 2.150 ACE (1.12; 2;60) |
| Cu | 0.410 DH (0.33; 0.48) | 0.460 BF (0.38; 0.57) | 0.890 EG (0.60; 1.22) | 0.960 AC (0.79; 1.15) |
| Mn | 4.110 BG (3.70; 4.71) | 2.10 FHJ (1.96; 2.32) | 9.010 ACE (7.44; 11.03) | 3.650 DI (3.31; 4.02) |
| Zn | 2.660 AC (1.43; 3.40) | 0.790 DH (0.16; 1.52) | 0.900 BF (0.56; 1.24) | 2.350 EG (2.18; 2.52) |
| Al | 4.790 B (2.17; 8.21) | 2.050 D (1.63; 2.46) | 0.350 F (0.25; 0.47) | 39.020 ACE (29.82; 48.77) |
| Cd | 0.030 E (0.02; 0.05) | 0.031 A (0.02; 0.04) | 0.011 BDF (0.01; 0.02) | 0.030 C (0.02; 0.04) |
| Pb | 0.058 E (0.04; 0.08) | 0.044 B (0.02; 0.09) | 0.026 DF (0.02; 0.04) | 0.073 AC (0.05; 0.10) |
| Total identified minerals | 1043.70 DbI (729.76; 1342.03) | 1346.82 BaG (1178.19; 1570.51) | 832.31 FHJ (725.23; 971.26) | 2408.90 ACE (2226.71; 2631.35) |
| Component (Min.; Max.) | Multifloral | Linden | Buckwheat | Honeydew |
|---|---|---|---|---|
| Fructose (g·100 g−1) | 39.548 BGI (36.72; 42.71) | 39.423 DHK (37.75; 41.40) | 48.945 ACE (46.49; 52.50) | 34.585 FJL (32.56; 36.72) |
| Glucose (g·100 g−1) | 31.381 (29.89; 32.69) | 28.265 (26.10; 30.10) | 29.543 (26.55; 31.24) | 28.100 (20.12; 33.87) |
| Sucrose (g·100 g−1) | 5.399 BG (4.02; 6.78) | 4.113 DI (3.22; 5.08) | 0.481 FHJ (0.35; 0.67) | 7.929 ACE (5.17; 10.46) |
| Maltose (g·100 g−1) | 1.012 D (0.34; 1.71) | 1.398 B (0.60; 2.71) | N/D - | 5.072 AC (1.48; 10.65) |
| Trehalose (g·100 g−1) | 0.319 (0.14; 0.50) | N/D - | N/D - | N/D - |
| Melezitose (g·100 g−1) | N/D - | N/D - | N/D - | 1.062 (0.13; 2.45) |
| Raffinose (g·100 g−1) | 0.610 (0.27; 1.45) | 1.036 a (0.07; 2.64) | 0.046 b (0.02; 0.08) | 0.653 (0.17; 1.11) |
| Total identified sugars (g·100 g−1) | 78.268 (73.74; 81.52) | 74.235 (70.58; 78.46) | 79.016 (73.99; 83.63) | 77.403 (69.55; 86.77) |
| F/G | 1.260 B (1.12; 1.38) | 1.398 b (1.29; 1.49) | 1.666 aAC (1.49; 1.77) | 1.269 D (1.08; 1.77) |
| Density (kg·m−3) | 1.418 A (1.414; 1.422) | 1.416 C (1.410; 1.423) | 1.415 a (1.411; 1.422) | 1.410 BDb (1.403; 1.416) |
| Specific rotation (°) | −15.803 FJL (−17.08; −14.98) | −6.903 BGI (−8.07; −5.47) | −12.247 DHK (−14.19; −10.32) | 2.085 ACE (0.66; 4.16) |
| pH | 4.647 C (3.97; 5.27) | 4.907 A (4.67; 5.26) | 3.811 BDF (3.71; 3.90) | 4.484 E (4.18; 4.84) |
| Free Acidity (meq acid·kg−1) | 18.751 Ea (15.66; 22.98) | 8.647 DFd (4.78; 12.62) | 20.031 AC (16.83; 23.11) | 13.977 Bbc (10.79; 19.81) |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Fructose | 0.888 | −0.281 | 0.188 | −0.095 |
| Glucose | 0.280 | 0.135 | −0.473 | −0.674 |
| Sucrose | −0.882 | 0.172 | −0.321 | −0.094 |
| Maltose | −0.824 | −0.196 | 0.064 | 0.188 |
| Trehalose | 0.143 | 0.385 | −0.830 | 0.179 |
| Melezitose | −0.723 | −0.362 | −0.043 | −0.214 |
| Raffinose | −0.410 | 0.525 | 0.185 | 0.424 |
| Ca | −0.455 | 0.675 | 0.506 | 0.016 |
| K | −0.934 | −0.222 | 0.089 | −0.027 |
| Mg | −0.709 | −0.442 | −0.436 | 0.191 |
| Fe | −0.934 | 0.074 | 0.205 | −0.037 |
| Cu | −0.221 | −0.889 | 0.199 | 0.037 |
| Mn | 0.642 | −0.720 | −0.040 | 0.080 |
| Zn | −0.432 | −0.009 | −0.813 | 0.092 |
| Al | −0.875 | −0.409 | −0.125 | −0.018 |
| Cd | −0.528 | 0.516 | −0.249 | −0.284 |
| Pb | −0.696 | 0.053 | −0.259 | 0.140 |
| Chlorogenic acid | −0.903 | −0.220 | −0.278 | 0.040 |
| Caffeic acid | 0.933 | −0.246 | 0.037 | 0.081 |
| Ferulic acid | 0.034 | −0.884 | −0.296 | −0.086 |
| Proline | −0.404 | −0.787 | 0.365 | −0.112 |
| Density | 0.516 | 0.464 | −0.242 | 0.094 |
| Specific rotation | −0.837 | −0.285 | 0.395 | −0.131 |
| pH | −0.417 | 0.731 | 0.007 | −0.235 |
| Free acidity | 0.482 | −0.514 | −0.586 | 0.117 |
| Variables | Root 1 | Root 2 | Root 3 |
|---|---|---|---|
| Fructose | −0.019 | 0.738 | 0.194 |
| Glucose | 0.012 | −0.462 | 0.112 |
| Sucrose | 0.823 | 0.229 | −0.923 |
| Maltose | −0.256 | 0.354 | −0.315 |
| Trehalose | −1.022 | 0.371 | −0.408 |
| Melezitose | −0.202 | 0.231 | 0.181 |
| Raffinose | 0.351 | −0.196 | 0.274 |
| Ca | −0.140 | −0.992 | 0.880 |
| K | 0.715 | 0.253 | −0.397 |
| Mg | 1.177 | −0.303 | −0.055 |
| Fe | 0.286 | −0.259 | 0.163 |
| Cu | 0.456 | −0.126 | 0.373 |
| Mn | −0.785 | 0.805 | −0.230 |
| Zn | 0.431 | 0.170 | −0.585 |
| Al | 0.103 | 0.283 | 0.402 |
| Cd | −0.099 | −0.308 | 0.385 |
| Pb | −0.277 | 0.211 | −0.525 |
| Chlorogenic acid | 1.096 | −0.159 | −0.210 |
| Caffeic acid | 0.011 | −0.175 | 0.251 |
| Ferulic acid | 0.297 | 0.711 | −0.155 |
| Proline | 0.255 | 0.062 | 0.704 |
| Density | −0.421 | 0.219 | −0.307 |
| Specific rotation | 0.626 | 0.486 | 0.188 |
| pH | 1.089 | −0.588 | −0.073 |
| Free acidity | 0.134 | −0.173 | 0.170 |
| Eigenvalue | 431.81 | 82.48 | 49.81 |
| Discrimination (%) | 76.55 | 14.62 | 8.83 |
| Cumulative (%) | 76.55 | 91.17 | 100.00 |
| Original Group | Predicted Classification (Number of Samples) | Correct Classification [%] | |||
| Multifloral | Lime | Buckwheat | Honeydew | ||
| Multifloral | 12 | 0 | 0 | 0 | 100 |
| Lime | 0 | 12 | 0 | 0 | 100 |
| Buckwheat | 0 | 0 | 12 | 0 | 100 |
| Honeydew | 0 | 0 | 0 | 12 | 100 |
| Total | 12 | 12 | 12 | 12 | 100 |
| Category | Type of Honey | Origin | Color | Aroma/Flavor | Location |
|---|---|---|---|---|---|
| light | Multifloral | Nectar | light golden, dark yellow to amber | very sweet aroma and mild taste | Podkarpacie |
| Linden | Nectar | spicy with light bitterness taste | Malopolska | ||
| dark | Buckwheat | Nectar | dark-tea, brown to black | distinctive aroma and spicy taste | Maloposka |
| Pine Honeydew | Honeydew | delicate sweet, slightly spicy, resinous taste | Podkarpacie |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarapatskyy, M.; Sowa, P.; Zaguła, G.; Dżugan, M.; Puchalski, C. Assessment of the Botanical Origin of Polish Honeys Based on Physicochemical Properties and Bioactive Components with Chemometric Analysis. Molecules 2021, 26, 4801. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26164801
Tarapatskyy M, Sowa P, Zaguła G, Dżugan M, Puchalski C. Assessment of the Botanical Origin of Polish Honeys Based on Physicochemical Properties and Bioactive Components with Chemometric Analysis. Molecules. 2021; 26(16):4801. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26164801
Chicago/Turabian StyleTarapatskyy, Maria, Patrycja Sowa, Grzegorz Zaguła, Małgorzata Dżugan, and Czesław Puchalski. 2021. "Assessment of the Botanical Origin of Polish Honeys Based on Physicochemical Properties and Bioactive Components with Chemometric Analysis" Molecules 26, no. 16: 4801. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26164801