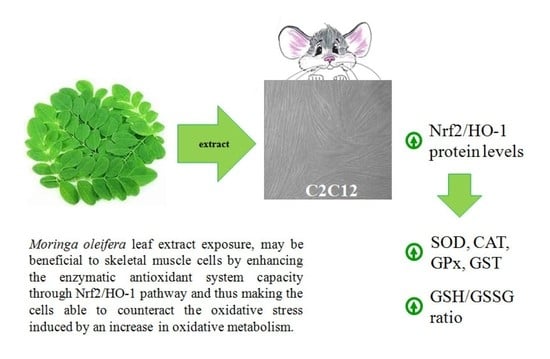
Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methanolic Extract of Moringa oleifera Leaves
2.2. Qualitative Profiling of MOLE Extract
2.3. Trolox® Equivalents Antioxidant Capacity
2.4. Cell Cultures
2.5. Glutathione Homeostasis
2.6. Preparation of Cell Homogenates and Western Blot Analysis
2.7. Enzymatic Activities
2.8. Lipid and Protein Oxidation
2.9. Statistical Analysis
3. Results
3.1. Metabolomic Fingerprint by UHPLC QTOF
3.2. In Vitro Antioxidant Capacity of MOLE
3.3. Evaluation of Redox Status after MOLE Treatment
3.4. Nrf2 and HO-1 Protein Expression
3.5. Evaluaion of Antioxidant Enzyme Activities after MOLE Treatment
3.6. Evaluation of Oxidative Damage after MOLE Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| MOLE | Moringa oleifera leaf extract |
| FAs | fatty acids |
| GLs | glucosinolates |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| GSH/GSSG | reduced to oxidized glutathione ratio |
| TAC | total antioxidant capacity |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| HO-1 | heme oxygenase-1 |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPx | glutathione peroxidase |
| GST | glutathione transferase |
| TBARS | thiobarbituric acid reactive substances |
| PrCar | protein carbonyls |
| ROS | reactive oxygen species |
References
- Ayoola, G.A.; Coker, H.A.B.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- Davinelli, S.; Bertoglio, J.C.; Zarrelli, A.; Pina, R.; Scapagnini, G. A randomized clinical trial evaluating the efficacy of an anthocyanin-maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll. Nutr. 2015, 34 (Suppl. 1), 28–33. [Google Scholar] [CrossRef] [Green Version]
- Posmontie, B. The medicinal qualities of Moringa oleifera. Holist Nurs. Pract. 2011, 25, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa Oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Nahar, S.; Faisal, F.M.; Iqbal, J.; Rahman, M.M.; Yusuf, M.A. Antiobesity activity of Moringa Oleifera leaves against high fat diet-induced obesity in rats. Int. J. Basic Clin. Pharmacol. 2016, 5, 1263–1268. [Google Scholar] [CrossRef]
- Bais, S.; Singh, G.S.; Sharma, R. Anti obesity and hypolipidemic activity of Moringa Oleifera leaves against high fat diet induced obesity in rats. Adv. Biol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Murillo, A.G.; Fernandez, M.L. The relevance of dietary polyphenols in cardiovascular protection. Curr. Pharmacol. Rev. Curr. Pharm. Des. 2017, 23, 2444–2452. [Google Scholar] [CrossRef]
- Pokorny, J. Introduction. In Antioxidant in Foods: Practical Applications; Pokorny, J., Yanishlieva, N., Gordon, N.H., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 1–3. [Google Scholar]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Farias, D.F.; Oliveira, J.T.D.A.; Carvalho, A.D.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008, 21, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Mishra, G.; Singh, P.; Verma, R.; Kumar, R.S.; Srivastava, S.; Khosla, R.L. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: An overview. Der. Pharm. Lett. 2011, 3, 141–164. [Google Scholar]
- Tejas, G.H.; Umang, J.H.; Payal, B.N.; Tusharbinu, D.R.; Pravin, T.R. A panoramic view on pharmacognostic, pharmacological, nutritional, therapeutic and prophylactic values of Moringa olifera Lam. Int. Res. J. Pharm. 2012, 3, 1–7. [Google Scholar]
- Lopez-Teros, V.; Ford, J.L.; Green, M.H.; Tang, G.; Grusak, M.A.; Quihui-Cota, L.; Muzhingi, T.; Paz-Cassini, M.; Astiazaran-Garcia, H. Use of a “Super-child” Approach to Assess the Vitamin A Equivalence of Moringa oleifera Leaves, Develop a Compartmental Model for Vitamin A Kinetics, and Estimate Vitamin A Total Body Stores in Young Mexican Children. J. Nutr. 2017, 147, 2356–2363. [Google Scholar] [CrossRef] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Cabrera, M.C.; Carretero, A.; Millan-Domingo, F.; Garcia-Dominguez, E.; Correas, A.G.; Olaso-Gonzalez, G.; Viña, J. Redox-related biomarkers in physical exercise. Redox Biol. 2021, 42, 101956. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; McArdle, A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J. Physiol. 2011, 589, 2139–2145. [Google Scholar] [CrossRef]
- Vasilaki, A.; Mansouri, A.; Van Remmen, H.; van der Meulen, J.H.; Larkin, L.; Richardson, A.G.; McArdle, A.; Faulkner, J.A.; Jackson, M.J. Free radical generation by skeletal muscle of adult and old mice: Effect of contractile activity. Aging Cell 2006, 5, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef]
- Duranti, G.; Maldini, M.; Crognale, D.; Sabatini, S.; Corana, F.; Horner, K.; Ceci, R. Moringa oleifera leaf extract influences oxidative metabolism in C2C12 myotubes through SIRT1-PPAR𝛼 pathway. Phytomed. Plus 2021, 1, 100014. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1𝛼 and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1𝛼. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ren, D.; Fedorova, J.; He, Z.; Li, J. SIRT1/SIRT3 Modulates Redox Homeostasis during Ischemia/Reperfusion in the Aging Heart. Antioxidants 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Kawai, Y.; Garduño, L.; Theodore, M.; Yang, J.; Arinze, I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 2011, 286, 7629–7640. [Google Scholar] [CrossRef] [Green Version]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Samimi, F.; Baazm, M.; Eftekhar, E.; Rajabi, S.; Goodarzi, M.T.; Jalali Mashayekhi, F. Possible antioxidant mechanism of coenzyme Q10 in diabetes: Impact on Sirt1/Nrf2 signaling pathways. Res. Pharm. Sci. 2019, 14, 524–533. [Google Scholar] [CrossRef]
- Wang, G.; Xie, X.; Yuan, L.; Qiu, J.; Duan, W.; Xu, B.; Chen, X. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors 2020, 46, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Duranti, G.; Ceci, R.; Sgrò, P.; Sabatini, S.; Di Luigi, L. Influence of the PDE5 inhibitor tadalafil on redox status and antioxidant defense system in C2C12 skeletal muscle cells. Cell Stress Chaperones 2017, 22, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Ceci, R.; Duranti, G.; Rossi, A.; Savini, I.; Sabatini, S. Skeletal muscle differentiation: Role of dehydroepiandrosterone sulfate. Horm. Metab. Res. 2011, 43, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Colamartino, M.; Santoro, M.; Duranti, G.; Sabatini, S.; Ceci, R.; Testa, A.; Padua, L.; Cozzi, R. Evaluation of levodopa and carbidopa antioxidant activity in normal human lymphocytes in vitro: Implication for oxidative stress in Parkinson’s disease. Neurotox. Res. 2015, 27, 106–117. [Google Scholar] [CrossRef]
- Testa, E.; Nardozi, D.; Antinozzi, C.; Faieta, M.; Di Cecca, S.; Caggiano, C.; Fukuda, T.; Bonanno, E.; Zhenkun, L.; Maldonado, A.; et al. H2AFX and MDC1 promote maintenance of genomic integrity in male germ cells. J. Cell Sci. 2018, 131, jcs214411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil reduces expression and release of IL-6 and IL-8 induced by reactive oxygen species in systemic sclerosis fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. [Google Scholar] [CrossRef]
- Di Luigi, L.; Duranti, G.; Antonioni, A.; Sgrò, P.; Ceci, R.; Crescioli, C.; Sabatini, S.; Lenzi, A.; Caporossi, D.; Del Galdo, F.; et al. The phosphodiesterase type 5 inhibitor sildenafil improves dna stability and redox homeostasis in systemic sclerosis fibroblasts exposed to reactive oxygen species. Antioxidants 2020, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Magi, F.; Dimauro, I.; Margheritini, F.; Duranti, G.; Mercatelli, N.; Fantini, C.; Ripani, F.R.; Sabatini, S.; Caporossi, D. Telomere length is independently associated with age, oxidative biomarkers, and sport training in skeletal muscle of healthy adult males. Free Radic. Res. 2018, 52, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Ceci, R.; Duranti, G.; Di Filippo, E.S.; Bondi, D.; Verratti, V.; Doria, C.; Caporossi, D.; Sabatini, S.; Dimauro, I.; Pietrangelo, T. Endurance training improves plasma superoxide dismutase activity in healthy elderly. Mech. Ageing Dev. 2019, 185, 111190. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Leonetti, A.; Pietropaoli, S.; Spinozzi, F.; Marcocci, L.; Amendola, R.; Cecconi, F.; Sabatini, S.; Mariottini, P.; et al. Adaptive responses of heart and skeletal muscle to spermine oxidase overexpression: Evaluation of a new transgenic mouse model. Free Radic. Biol. Med. 2017, 103, 216–225. [Google Scholar] [CrossRef]
- Colamartino, M.; Duranti, G.; Ceci, R.; Sabatini, S.; Testa, A.; Cozzi, R. A multi-biomarker analysis of the antioxidant efficacy of parkinson’s disease therapy. Toxicol. In Vitro 2017, 47, 1–7. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tis- sues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Fahey, J.W.; Olson, M.E.; Stephenson, K.K.; Wade, K.L.; Chodur, G.M.; Odee, D.; Nouman, W.; Massiah, M.; Alt, J.; Egner, P.A.; et al. The diversity of chemoprotective glucosinolates in moringaceae (Moringa spp.). Sci. Rep. 2018, 8, 7994. [Google Scholar] [CrossRef] [Green Version]
- Heinzel, F.R.; Luo, Y.; Dodoni, G.; Boengler, K.; Petrat, F.; Di Lisa, F.; de Groot, H.; Schulz, R.; Heusch, G. Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc. Res. 2006, 71, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Niess, A.M.; Simon, P. Response and adaptation of skeletal muscle to exercise--the role of reactive oxygen species. Front. Biosci. 2007, 12, 4826–4838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [Green Version]
- Antonioni, A.; Fantini, C.; Dimauro, I.; Caporossi, D. Redox homeostasis in sport: Do athletes really need antioxidant support? Res. Sports Med. 2019, 27, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Deng, Y.; Liao, L.; Zhou, M.; Peng, C.; Li, Y. Quercetin as a protective agent for liver diseases: A comprehensive descriptive review of the molecular mechanism. Phytother. Res. 2021, 22. [Google Scholar] [CrossRef]
- Aaseth, J.; Alexander, J.; Alehagen, U. Coenzyme Q10 supplementation—In ageing and disease. Mech. Ageing Dev. 2021, 197, 111521. [Google Scholar] [CrossRef] [PubMed]
- Hyun, D.H. Insights into the New Cancer Therapy through Redox Homeostasis and Metabolic Shifts. Cancers 2020, 12, 1822. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Kim, C.S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1. J. Med. Food. 2018, 21, 551–559. [Google Scholar] [CrossRef]
- Suliman, H.B.; Carraway, M.S.; Tatro, L.G.; Piantadosi, C.A. A new activating role for CO in cardiac mitochondrial biogenesis. J. Cell Sci. 2007, 120, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, M.A.; Carraway, M.S.; Piantadosi, C.A.; Reynolds, C.M.; Cherry, A.D.; Wester, T.E.; Natoli, M.J.; Massey, E.W.; Moon, R.E.; Suliman, H.B. Carbon monoxide, skeletal muscle oxidative stress, and mitochondrial biogenesis in humans. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H392–H399. [Google Scholar] [CrossRef] [Green Version]
- Islam, H.; Bonafiglia, J.T.; Turnbull, P.C.; Simpson, C.A.; Perry, C.G.R.; Gurd, B.J. The impact of acute and chronic exercise on Nrf2 expression in relation to markers of mitochondrial biogenesis in human skeletal muscle. Eur. J. Appl. Physiol. 2020, 120, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Ndisang, J.F. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators Inflamm. 2010, 2010, 359732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.S.; Han, M.H.; Kim, G.Y.; Kim, C.M.; Kim, B.W.; Hwang, H.J.; Hyun, Y. Nrf2-mediated HO-1 induction contributes to antioxidant capacity of a Schisandrae Fructus ethanol extract in C2C12 myoblasts. Nutrients 2014, 6, 5667–5678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.S.; Choi, I.W.; Han, M.H.; Lee, D.S.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. The Cytoprotective Effect of Petalonia binghamiae Methanol Extract against Oxidative Stress in C2C12 Myoblasts: Mediation by Upregulation of Heme Oxygenase-1 and Nuclear Factor-Erythroid 2 Related Factor 2. Mar. Drugs 2015, 13, 2666–2679. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.O.; Son, Y.; Lee, J.H.; Cheong, Y.K.; Park, S.H.; Chung, H.T.; Pae, H.O. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep. 2015, 12, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Total Antioxidant Capacity: Appraisal of a Concept. J. Nutr. 2007, 137, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta 2014, 1840, 931–934. [Google Scholar] [CrossRef]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P.; et al. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010, 79, 1141. [Google Scholar] [CrossRef] [Green Version]
- Förster, N.; Mewis, I.; Glatt, H.; Haack, M.; Brigelius-Flohé, R.; Schreiner, M.; Ulrichs, C. Characteristic single glucosinolates from Moringa oleifera: Induction of detoxifying enzymes and lack of genotoxic activity in various model systems. Food Funct. 2016, 7, 4660–4674. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Basu, A.; Singha Roy, S.; Patra, A.R.; Bhattacharya, S. Attenuation of doxorubicin-induced cardiotoxicity and genotoxicity by an indole-based natural compound 3,3’-diindolylmethane (DIM) through activation of Nrf2/ARE signaling pathways and inhibiting apoptosis. Free Radic. Res. 2017, 51, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Hood, D.A.; Gurd, B.J. Looking beyond PGC-1 𝛼: Emerging regulators of exercise-induced skeletal muscle mitochondrial biogenesis and their activation by dietary compounds. Appl. Physiol. Nutr. Metab. 2020, 45, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, F.; Ditroilo, M.; Felici, F.; Duranti, G.; De Vito, G.; Sabatini, S.; Sacchetti, M.; Bazzucchi, I. The acute effect of Quercetin on muscle performance following a single resistance training session. Eur. J. Appl. Physiol. 2018, 118, 1021–1031. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sabatini, S.; Sgrò, P.; Di Luigi, L.; Sacchetti, M. Quercetin supplementation improves neuromuscular function recovery from muscle damage. Nutrients 2020, 12, 2850. [Google Scholar] [CrossRef]
- Edenfield, K.M. Sports Supplements: Pearls and Pitfalls. Prim. Care 2020, 47, 37–48. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]






| Components | Relative Amounts (%) |
|---|---|
| Glucomoringin | 23.21 |
| 4-O-acetylrhamnopyranosyloxybenzylGS/7.67 | 22.16 |
| Isoquercitrin | 7.39 |
| Rutin | 5.28 |
| Quercetin-O-β-d-glucose-acetate isomer/11.53 | 4.92 |
| Neochlorogenic acid | 4.57 |
| Chlorogenic acid | 4.57 |
| Cryptochlorogenic acid | 4.57 |
| Astragalin/Luteoloside | 4.22 |
| 4-O-acetylrhamnopyranosyloxybenzylGS/5.99 | 3.17 |
| Quinic acid | 2.81 |
| Kaempferol-O-rutinoside | 2.46 |
| 4-O-acetylrhamnopyranosylosxybenzylGS/6.41 | 2.11 |
| Vitexin | 2.11 |
| Isovitexin | 2.11 |
| Quercetin-O-β-d-glucose-acetate isomer/11.98 | 1.06 |
| Nepetin 7-glucoside | 0.88 |
| Quercetin-O-β-d-glucose-acetate isomer/12.34 | 0.70 |
| Orientin | 0.53 |
| 4-O-acetylglucopyranosyloxybenzylGS/6.97 | 0.18 |
| Esculin | 0.18 |
| Isorhamnetin-O-neohespeidoside | 0.18 |
| Glucosoonjnain | 0.18 |
| Quercetin | 0.04 |
| Citric acid | 0.04 |
| Sinalbin | 0.04 |
| Kaempferol | 0.04 |
| Kaempferol 3-O-(3″,4″-di-O-acetyl-α-l-rhamnopyranoside) | 0.04 |
| Isorhamnetin | 0.04 |
| Vitamin B2 | 0.04 |
| Quercetin-di-O-glucoside | 0.04 |
| Pueranin | 0.04 |
| 4-O-acetylglucopyranosyloxybenzylGS/5.34 | 0.04 |
| 4-O-acetylglucopyranosyloxybenzylGS/6.16 | 0.04 |
| Ferulic/Isoferulic acid | 0.04 |
| Protocatechuic acid | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duranti, G.; Maldini, M.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Ceci, R. Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells. Molecules 2021, 26, 5041. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26165041
Duranti G, Maldini M, Crognale D, Horner K, Dimauro I, Sabatini S, Ceci R. Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells. Molecules. 2021; 26(16):5041. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26165041
Chicago/Turabian StyleDuranti, Guglielmo, Mariateresa Maldini, Domenico Crognale, Katy Horner, Ivan Dimauro, Stefania Sabatini, and Roberta Ceci. 2021. "Moringa oleifera Leaf Extract Upregulates Nrf2/HO-1 Expression and Ameliorates Redox Status in C2C12 Skeletal Muscle Cells" Molecules 26, no. 16: 5041. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26165041