Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections—An In Vitro and In Silico Perspective
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Plant Extracts for Assays
2.2. Antimicrobial Activity
2.3. Antibiotic Potentiating Activity
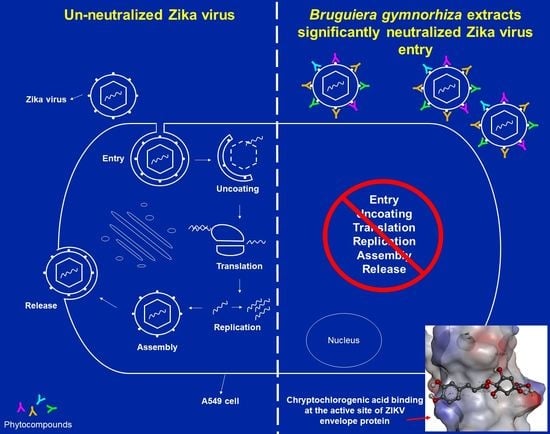
2.4. BRA and BFA Extracts Inhibit ZIKV Infection at Non-Cytotoxic Concentrations
2.5. Viral Inactivation Assay Shows That BRA and BFA Prevent ZIKV Entry in A549 Cells
2.6. In Silico Docking Analysis
2.7. In Silico ADME Analysis of Cryptochlorogenic Acid
3. Materials and Methods
3.1. Plant Materials
3.2. Preparation of Extracts
3.3. Antimicrobial Assay
3.4. Antibiotic Potentiating Assay
3.5. Cell Cultures
3.6. MTT Assay
3.7. Virus Inactivation Assay
3.8. Flow Cytometry Assay
3.9. In Silico Docking Analysis
3.10. In Silico ADME Analysis of Cryptochlorogenic Acid
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| BFA | Bruguiera fruit aqueous |
| BLA | Bruguiera leaf aqueous |
| BRA | Bruguiera root aqueous |
| BTA | Bruguiera twig aqueous |
| BFD | Bruguiera fruit decoction |
| BLD | Bruguiera leaf decoction |
| BRD | Bruguiera root decoction |
| BTD | Bruguiera twig decoction |
| BFE | Bruguiera fruit ethyl acetate |
| BLE | Bruguiera leaf ethyl acetate |
| BRE | Bruguiera root ethyl acetate |
| BTE | Bruguiera twig ethyl acetate |
| BFM | Bruguiera fruit methanolic |
| BLM | Bruguiera leaf methanolic |
| BRM | Bruguiera root methanolic |
| BTM | Bruguiera twig methanolic |
References
- Dos Santos Ramos, M.A.; dos Santos, K.C.; da Silva, P.B.; de Toledo, L.G.; Marena, G.D.; Rodero, C.F.; de Camargo, B.A.F.; Fortunato, G.C.; Bauab, T.M.; Chorilli, M. Nanotechnological strategies for systemic microbial infections treatment: A review. Int. J. Pharm. 2020, 589, 119780. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Sadeer, N.B.; Mahomoodally, F. Antibiotic potentiation of natural products: A promising target to fight pathogenic bacteria. Curr. Drug Targets 2020, 21, 1–17. [Google Scholar]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 2019, 10, 277–290. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, F. Antimicrobial, antibiotic potentiating activity and phytochemical profile of essential oils from exotic and endemic medicinal plants of Mauritius. Ind. Crop. Prod. 2015, 71, 197–204. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Alves, E.F.; Santos, B.S.; Sobral de Souza, C.E.; Alencar Ferreira, J.V.d.; Santos de Lavor, A.K.L.; Figueredo, F.G.; Ferreira de Lima, L.; Vieira dos Santos, F.A.; Neves Peixoto, F.S. Biological activities and chemical characterization of Cordia verbenacea DC. as tool to validate the ethnobiological usage. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, J.J.; Sousa, C.P.; Araruna, M.K.; Silva, M.K.; Portelo, A.C.; Lopes, J.C.; Carvalho, V.R.; Figueredo, F.G.; Bitu, V.C.; Coutinho, H.D. Antibacterial and modifying-antibiotic activities of the essential oils of Ocimum gratissimum L. and Plectranthus amboinicus L. Eur. J. Integr. Med. 2015, 7, 151–156. [Google Scholar] [CrossRef]
- Akrami, K.M.; de Nogueira, B.M.F.; do Rosário, M.S.; de Moraes, L.; Cordeiro, M.T.; Haddad, R.; Gomes, L.N.; de Pádua Carvalho, I.; Dos Reis Pimentel, E.; de Jesus Silva, J.; et al. The re-emergence of Zika in Brazil in 2020: A case of Guillain Barré Syndrome during the low season for arboviral infections. J. Travel Med. 2020, 27, 165. [Google Scholar] [CrossRef]
- Casas, X. New Zika Cases in Brazil Overshadowed by COVID-19. Available online: https://www.hrw.org/news/2020/05/28/new-zika-cases-brazil-overshadowed-COVID-19 (accessed on 6 January 2021).
- Reis, A.C.C.; Silva, B.M.; de Moura, H.M.M.; Pereira, G.R.; Brandão, G.C. Anti-Zika virus activity and chemical characterization by ultra-high-performance liquid chromatography (UPLC-DAD-UV-MS) of ethanol extracts in Tecoma species. BMC Complement. Med. Ther. 2020, 20, 246. [Google Scholar] [CrossRef]
- Bujalowski, P.J.; Bujalowski, W.; Choi, K.H. Identification of the viral RNA promoter stem loop A (SLA)-binding site on Zika virus polymerase NS5. Sci. Rep. 2020, 10, 13306. [Google Scholar] [CrossRef]
- Sarkar, S.; Gardner, L. Zika: The cost of neglect. Palgrave Commun. 2016, 2, 16060. [Google Scholar] [CrossRef]
- Byler, K.G.; Ogungbe, I.V.; Setzer, W.N. In-silico screening for anti-Zika virus phytochemicals. J. Mol. Graph. Model. 2016, 69, 78–91. [Google Scholar] [CrossRef]
- Sousa, F.T.G.; Nunes, C.; Romano, C.M.; Sabino, E.C.; González-Cardenete, M.A. Anti-Zika virus activity of several abietane-type ferruginol analogues. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e97. [Google Scholar] [CrossRef]
- Gao, Y.; Tai, W.; Wang, N.; Li, X.; Jiang, S.; Debnath, A.K.; Du, L.; Chen, S. Identification of novel natural products as effective and broad-spectrum anti-Zika virus inhibitors. Viruses 2019, 11, 1019. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque de Oliveira Mendes, L.; Ponciano, C.S.; Depieri Cataneo, A.H.; Wowk, P.F.; Bordignon, J.; Silva, H.; Vieira de Almeida, M.; Ávila, E.P. The anti-Zika virus and anti-tumoral activity of the citrus flavanone lipophilic naringenin-based compounds. Chem. Biol. Interact. 2020, 331, 109218. [Google Scholar] [CrossRef]
- Acquadro, S.; Civra, A.; Cagliero, C.; Marengo, A.; Rittà, M.; Francese, R.; Sanna, C.; Bertea, C.; Sgorbini, B.; Lembo, D.; et al. Punica granatum leaf ethanolic extract and ellagic acid as inhibitors of Zika virus infection. Planta Med. 2020, 86, 1363–1374. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Kannan, R.R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, phytochemistry, and global distribution of mangroves―A comprehensive review. Mar. Drugs 2019, 17, 231. [Google Scholar]
- Lindsay, J.A. Hospital-associated MRSA and antibiotic resistance-what have we learned from genomics? Int. J. Med. Microbiol. 2013, 303, 318–323. [Google Scholar] [CrossRef]
- CDC, Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus Aureus (MRSA) Infections. Available online: https://www.cdc.gov/mrsa/index.html#:~:text=Methicillin%2Dresistant%20Staphylococcus%20aureus%20(MRSA),-Related%20Pages&text=Staph%20bacteria%20are%20usually%20harmless,of%20resistance%20to%20some%20antibiotics (accessed on 7 January 2021).
- Muir, W.W.; Sams, R.A. Pharmacologic principles and pain: Pharmacokinetics and pharmacodynamics. In Handbook of Veterinary Pain Management; Gaynor, J.S., Muir, W.W., Eds.; Mosby: Saint Louis, MO, USA, 2009; pp. 113–140. [Google Scholar]
- Cadelis, M.M.; Pike, E.I.; Kang, W.; Wu, Z.; Bourguet-Kondracki, M.-L.; Blanchet, M.; Vidal, N.; Brunel, J.M.; Copp, B.R. Exploration of the antibiotic potentiating activity of indolglyoxylpolyamines. Eur. J. Med. Chem. 2019, 183, 111708. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Bréchot, N.; Trouillet, J.-L.; Chastre, J. Antibiotic stewardship in the intensive care unit. Crit. Care 2014, 18, 480. [Google Scholar] [CrossRef] [Green Version]
- Latif, A.; Amer, H.M.; Hamad, M.E.; Alarifi, S.A.R.; Almajhdi, F.N. Medicinal plants from Saudi Arabia and Indonesia: In vitro cytotoxicity evaluation on Vero and Hep-2 cells. J. Med. Plant Res. 2014, 8, 1065–1073. [Google Scholar]
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 7 January 2021).
- Bobrowski, T.; Chen, L.; Eastman, R.T.; Itkin, Z.; Shinn, P.; Chen, C.; Guo, H.; Zheng, W.; Michael, S.; Simeonov, A.; et al. Discovery of synergistic and antagonistic drug combinations against SARS-CoV-2 in vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, Á.; Jiménez de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.C. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses West Nile virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef]
- Haddad, J.G.; Grauzdytė, D.; Koishi, A.C.; Viranaicken, W.; Venskutonis, P.R.; Nunes Duarte dos Santos, C.; Desprès, P.; Diotel, N.; El Kalamouni, C. The geraniin-rich extract from Reunion Island endemic medicinal plant Phyllanthus phillyreifolius inhibits Zika and Dengue Virus infection at non-toxic effect doses in Zebrafish. Molecules 2020, 25, 2316. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhang, B.Y.; Qiu, L.P.; Guan, R.F.; Ye, Z.H.; Yu, X.P. Structure properties and mechanisms of action of naturally originated phenolic acids and their derivatives against human viral infections. Curr. Med. Chem. 2017, 24, 4279–4302. [Google Scholar] [CrossRef]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.-Q.; Musyoki, Abednego, M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika virus envelope protein and its complex with a flavivirus broadly protective antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef]
- Balta, I.; Stef, L.; Pet, I.; Ward, P.; Callaway, T.; Ricke, S.C.; Gundogdu, O.; Corcionivoschi, N. Antiviral activity of a novel mixture of natural antimicrobials, in vitro, and in a chicken infection model in vivo. Sci. Rep. 2020, 10, 16631. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; de Arruda, T.B.; Suzukawa, A.A.; Bortot, L.O.; Dias-Baruffi, M.; Verri, W.A.; et al. The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.C.; Chen, M.C.; Liu, S.; Callahan, V.M.; Bracci, N.R.; Lehman, C.W.; Dahal, B.; de la Fuente, C.L.; Lin, C.C.; Wang, T.T.; et al. Phloretin inhibits Zika virus infection by interfering with cellular glucose utilisation. Int. J. Antimicrob. Agents 2019, 54, 80–84. [Google Scholar] [CrossRef]
- Zanello, P.R.; Koishi, A.C.; Rezende Júnior Cde, O.; Oliveira, L.A.; Pereira, A.A.; de Almeida, M.V.; Duarte dos Santos, C.N.; Bordignon, J. Quinic acid derivatives inhibit dengue virus replication in vitro. Virol. J. 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Demerdash, A.; Metwaly, A.; Hassan, A.; Abd El-Aziz, T.; Elkaeed, E.; Eissa, I.; Arafa, R.; Stockand, J. Comprehensive virtual screening of the antiviral potentialities of marine polycyclic guanidine alkaloids against SARS-CoV-2 (COVID-19). Biomolecules 2021, 11, 460. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaney, J.S. ESOL: Estimating aqueous solubility directly from molecular structure. J. Chem. Inform. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the general solubility equation: In silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 2012, 52, 420–428. [Google Scholar] [CrossRef]
- Koc, Z.E.; Uysal, A. Investigation of novel monopodal and dipodal oxy-Schiff base triazine from cyanuric chloride: Structural and antimicrobial studies. J. Macromol. Sci. A 2016, 53, 111–115. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Rocchetti, G.; Senizza, B.; Montesano, D.; Zengin, G.; Uysal, A.; Jeewon, R.; Lucini, L.; Mahomoodally, M.F. Untargeted metabolomic profiling, multivariate analysis and Biological evaluation of the true mangrove (Rhizophora mucronata Lam.). Antioxidants 2019, 8, 489. [Google Scholar] [CrossRef] [Green Version]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Bouhdid, S.; Balouiri, M.; Lalami, A.E.O.; Moja, S.; Chahdi, F.O.; Greche, H. Chemical composition of Citrus aurantium L. leaves and zest essential oils, their antioxidant, antibacterial single and combined effects. J. Chem. Pharm. Res. 2015, 7, 78–84. [Google Scholar]
- Seebaluck-Sandoram, R.; Lall, N.; Fibrich, B.; van Staden, A.B.; Mahomoodally, F. Antibiotic-potentiating activity, phytochemical profile, and cytotoxicity of Acalypha integrifolia Willd. (Euphorbiaceae). J. Herb. Med. 2018, 11, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Frumence, E.; Roche, M.; Krejbich-Trotot, P.; El-Kalamouni, C.; Nativel, B.; Rondeau, P.; Missé, D.; Gadea, G.; Viranaicken, W.; Desprès, P. The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-β production and apoptosis induction. Virology 2016, 493, 217–226. [Google Scholar] [CrossRef]
- Gadea, G.; Bos, S.; Krejbich-Trotot, P.; Clain, E.; Viranaicken, W.; El-Kalamouni, C.; Mavingui, P.; Desprès, P. A robust method for the rapid generation of recombinant Zika virus expressing the GFP reporter gene. Virology 2016, 497, 157–162. [Google Scholar] [CrossRef]
- Clain, E.; Sinigaglia, L.; Koishi, A.C.; Gorgette, O.; Gadea, G.; Viranaicken, W.; Krejbich-Trotot, P.; Mavingui, P.; Desprès, P.; dos Santos, C.N.D. Extract from Aphloia theiformis, an edible indigenous plant from Reunion Island, impairs Zika virus attachment to the host cell surface. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Clain, E.; Haddad, J.G.; Koishi, A.C.; Sinigaglia, L.; Rachidi, W.; Desprès, P.; N Duarte dos Santos, C.; Guiraud, P.; Jouvenet, N.; El Kalamouni, C. The polyphenol-rich extract from psiloxylon mauritianum, an endemic medicinal plant from Reunion Island, inhibits the early stages of dengue and Zika virus infection. Int. J. Mol. Sci. 2019, 20, 1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, J.G.; Picard, M.; Bénard, S.; Desvignes, C.; Desprès, P.; Diotel, N.; El Kalamouni, C. Ayapana triplinervis essential oil and its main component thymohydroquinone dimethyl ether inhibit Zika virus at doses devoid of toxicity in Zebrafish. Molecules 2019, 24, 3447. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E. Gaussian 09, revision B.01. Available online: http://0-www-rsc-org.brum.beds.ac.uk/suppdata/ob/c4/c4ob00537f/c4ob00537f2.pdf (accessed on 23 January 2021).







| Method | Plant Part | Code | Yield (%) |
|---|---|---|---|
| Decoction | Leaf | BLD | 18.70 |
| Root | BRD | 7.38 | |
| Twig | BTD | 7.78 | |
| Fruit | BFD | 8.24 | |
| Maceration: Water | Leaf | BLA | 11.04 |
| Root | BRA | 4.28 | |
| Twig | BTA | 4.26 | |
| Fruit | BFA | 11.12 | |
| Maceration: Ethyl Acetate | Leaf | BLE | 1.88 |
| Root | BRE | 0.46 | |
| Twig | BTE | 1.06 | |
| Fruit | BFE | 0.66 | |
| Maceration: Methanol | Leaf | BLM | 12.62 |
| Root | BRM | 9.60 | |
| Twig | BTM | 8.26 | |
| Fruit | BFM | 14.28 |
| Strains | MIC50 Values of B. gymnorhiza Extracts (mg/mL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BLM | BRM | BTM | BFM | BLE | BRE | BTE | BFE | BLA | BRA | BTA | BFA | BLD | BRD | BTD | BFD | Gentamicin (µg/mL) | |
| Escherichia coli ATCC 25922 | - | 1.56 | - | - | - | 0.78 | 0.19 | 0.78 | - | - | - | 6.25 | - | - | - | 3.12 | 0.312 |
| Pseudomonas aeruginosa ATCC 27853 | 0.78 | - | 0.78 | 0.78 | 0.78 | 0.78 | 0.19 | 0.39 | - | - | - | 3.12 | - | 1.56 | 3.25 | 0.78 | 0.039 |
| Klebsiella pneumoniae ATCC 70603 | - | 1.56 | - | 1.56 | - | 1.56 | 0.39 | 0.78 | - | - | - | - | - | - | - | 1.56 | 1.25 |
| Staphylococcus aureus ATCC 43300 (MRSA) | 3.12 | 0.39 | 0.78 | 0.39 | 3.12 | 0.78 | 0.19 | 0.78 | - | 0.39 | 6.25 | 0.39 | 6.25 | 0.39 | 0.78 | 1.56 | 0.078 |
| Salmonella enteritidis ATCC 13076 | - | 1.56 | - | 1.56 | - | 1.56 | 0.39 | 0.78 | - | - | - | 3.12 | - | 3.12 | - | 1.56 | 0.078 |
| Sarcina lutea ATCC 9341 | 1.56 | - | - | 1.56 | - | 1.56 | 0.39 | 0.78 | - | - | - | 3.12 | - | 3.12 | - | 1.56 | 0.039 |
| Proteus mirabilis ATCC 25933 | - | 0.78 | 1.56 | 0.39 | - | 0.78 | 0.39 | 1.56 | - | 3.12 | - | 1.56 | - | 0.78 | 3.12 | 1.56 | 0.312 |
| Bacillus cereus ATCC 11778 | - | - | - | - | - | 1.56 | 0.39 | 0.78 | - | - | - | - | - | - | - | 3.12 | <0.039 |
| Candida albicans ATCC 26555 | - | - | - | 1.56 | - | 1.56 | 0.39 | 0.78 | - | - | - | - | - | 6.25 | - | 3.12 | 0.312 |
| BTE/Antibiotic Combination (1:1) | E. coli | P. aeruginosa | MRSA | |||
|---|---|---|---|---|---|---|
| FIC | FICI | FIC | FICI | FIC | FICI | |
| BTE | 0.13 | 1.13 b | 0.25 | 2.25 b | 0.13 | 0.62 a |
| CIP | 1 | 2 | 0.49 | |||
| BTE | 1 | 5 c | 1 | 1.25 b | 0.26 | 2.26 b |
| CHL | 4 | 0.25 | 2 | |||
| BTE | 0.26 | 2.26 b | 0.13 | 0.62 a | 0.26 | 1.26 b |
| STR | 2 | 0.49 | 1 | |||
| Extract | CC50 (µMg/mL) a | IC50 (µg/mL) b | SI c |
|---|---|---|---|
| BFA | 520 | 130 | 4.0 |
| BRA | 470 | 140 | 3.3 |
| Compound | Binding Free Energy | Inhibition Constant | Protein-Ligand Interactions |
|---|---|---|---|
| Brugierol | −3.11 | 5.3 nM | Lys251 (HB), Asn103, Ala250, His249, Arg252 |
| Bruguierol A | −4.31 | 689.9 µM | Gly102 (HB), Arg252, His249, Lys251, Ala250 (HB) and (π- π) |
| Cryptochlorogenic Acid | −5.44 | 102.1 µM | Asp98 (HB), Ala248 (HB), Ala250 (HB), Lys251 (π- π), Val97, Asn103, His249. |
| Citric acid | −3.31 | 3.75 mM | His249 (HB), Ala248 (HB), Asn103 (HB), Asp98 (HB), Val97, Ala250. |
| Naringenin | −5.07 | 192.7 µM | Lys251 (HB), Val97, Asp98 (HB), Ala250, Asn103, His249. |
| Neochlorogenic Acid | −4.07 | 1.1 mM | Asp98 (HB), Val97, Ala250, Asn103, Arg99, Lys110, Gly109, Phe108. |
| Phloretin | −4.65 | 393.5 µM | Ala250 (HB), Lys251 (HB), Ala248 (HB), Val97, His249, Asn103, Gly102. |
| Procyanidin B | −4.57 | 447.6 µM | Ala248 (HB), Ala250 (HB), Arg99 (HB), Gly102 (HB), Asn103 (HB), Arg252. |
| Procyanidin C | −4.09 | 1.0 mM | Ala248 (HB), His249 (HB), Asp98 (HB), Lys110, Val97, Ala250, Asn103, Gly102. |
| Quinic acid | −4.35 | 644.0 µM | Asp98 (HB), Ala248 (HB), His249 (HB), Val97, Lys110, Asn103. |
| Physicochemical Properties | Lipophilicity | Water Solubility | |||
|---|---|---|---|---|---|
| Formula | C16H18O9 | Log Po/w (iLOGP) | 1.23 | Log S (ESOL) = −1.62 | Very soluble |
| MW | 354.31 g/mol | Log Po/w (XLOGP3) | −0.42 | Log S (Ali) = −2.58 | Soluble |
| No. of heavy atoms | 25 | Log Po/w (WLOGP) | −0.75 | Log S (SILICOS-IT) = 0.40 | Soluble |
| No. aromatic heavy atoms | 6 | Log Po/w (MLOGP) | −1.05 | ||
| Fraction Csp3 | 0.38 | Log Po/w (SILICOS-IT) | −0.6 | ||
| No. rotatable bonds | 5 | Mean Log Po/w | −0.32 | ||
| No. H-bond acceptors | 9 | ||||
| No. H-bond donors | 6 | ||||
| Molar refractivity | 83.5 | ||||
| TPSA | 164.75 Å2 | ||||
| Pharmacokinetics | Drug Likeness | Medicinal Chemistry | |||
| GI absorption | Low | Lipinski | Yes; 1 violation: NH or OH > 5 | PAINS | 1 alert: catechol_A |
| BBB permeant | No | Ghose | No; 1 violation: WLOGP < −0.4 | Lead-likeness | No; 1 violation: MW > 350 |
| P-gp substrate | No | Veber | No; 1 violation: TPSA > 140 | Synthetic accessibility | 4.13 |
| CYP1A2 inhibitor | No | Egan | No; 1 violation: TPSA > 131.6 | ||
| CYP2C19 inhibitor | No | Bioavailability Score | 0.11 | ||
| CYP2C9 inhibitor | No | ||||
| CYP2D6 inhibitor | No | ||||
| CYP3A4 inhibitor | No | ||||
| Log Kp (skin permeation) | −8.76 cm/s | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi Sadeer, N.; Haddad, J.G.; Oday Ezzat, M.; Desprès, P.; Abdallah, H.H.; Zengin, G.; Uysal, A.; El Kalamouni, C.; Gallo, M.; Montesano, D.; et al. Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections—An In Vitro and In Silico Perspective. Molecules 2021, 26, 5768. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195768
Bibi Sadeer N, Haddad JG, Oday Ezzat M, Desprès P, Abdallah HH, Zengin G, Uysal A, El Kalamouni C, Gallo M, Montesano D, et al. Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections—An In Vitro and In Silico Perspective. Molecules. 2021; 26(19):5768. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195768
Chicago/Turabian StyleBibi Sadeer, Nabeelah, Juliano G. Haddad, Mohammed Oday Ezzat, Philippe Desprès, Hassan H. Abdallah, Gokhan Zengin, Ahmet Uysal, Chaker El Kalamouni, Monica Gallo, Domenico Montesano, and et al. 2021. "Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections—An In Vitro and In Silico Perspective" Molecules 26, no. 19: 5768. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26195768