Red Light-Emitting Water-Soluble Luminescent Iridium-Containing Polynorbornenes: Synthesis, Characterization and Oxygen Sensing Properties in Biological Tissues In Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Polymers P1–P4
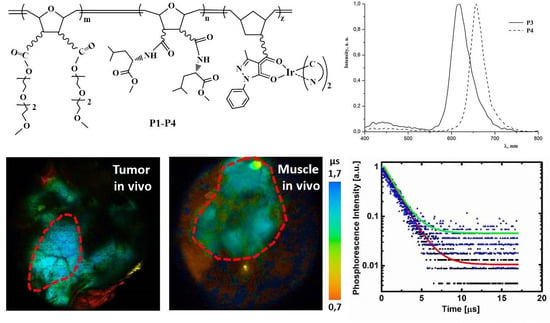
2.2. Photophysical Properties of Polymers P1–P4
2.3. Biological Properties of Polymers P1–P4
3. Materials and Methods
3.1. Instruments and Characterization
3.2. Synthesis of Polymers
3.3. In Vitro Cytotoxicity Assay and Cellular Uptake Study
3.4. In Vivo Animal Study
3.5. Immunohistochemical (IHC) Staining for Hypoxia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wong, P.T.; Choi, S.K. Mechanisms of drug release in nanotherapeutic delivery systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef]
- Li, N.; Zhao, L.; Qi, L.; Li, Z.; Luan, Y. Polymer assembly: Promising carriers as co-delivery systems for cancer therapy. Prog. Polym. Sci. 2016, 58, 1–26. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Houdaihed, L.; Evans, J.C.; Allen, C. Overcoming the road blocks; advancement of block copolymer micelles for cancer therapy in the clinic. Mol. Pharm. 2017, 14, 2503–2517. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Lu, X.; Fan, Q.; Zhang, Z.; Song, W.; Li, B.; Huang, L.; Peng, J.; Huang, W. Water-soluble iridium(III)-containing conjugated polyelectrolytes with weakened energy transfer properties for multicolor protein sensing applications. Macromolecules 2011, 44, 8763–8770. [Google Scholar] [CrossRef]
- Fang, X.; Ju, B.; Liu, Z.; Wang, F.; Xi, G.; Sun, Z.; Chen, H.; Sui, C.; Wang, M.; Wu, C. Compact conjugated polymer dots with covalently incorporated metalloporphyrins for hypoxia bioimaging. ChemBioChem 2019, 20, 521–525. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, W.; Cao, G.; Chen, Y.; Ma, Y.; Huang, Y.; Liu, X.; Xu, W.; Zhao, Q.; Huang, W. Smart poly(N-isopropylacrylamide) containing iridium(III) complexes as water-soluble phosphorescent probe for sensing and bioimaging of homocysteine and cysteine. Macromol. Rapid Commun. 2013, 34, 81–86. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Mao, H.; Wu, W.; Liu, B.; Jiang, X. Hypoxia-specific ultrasensitive detection of tumours and cancer cells in vivo. Nat. Commun. 2015, 6, 5834. [Google Scholar] [CrossRef]
- Sankaran, N.B.; Rys, A.Z.; Nassif, R.; Nayak, M.K.; Metera, K.; Chen, B.; Bazzi, H.S.; Sleiman, H.F. Ring-opening metathesis polymers for biodetection and signal amplification: Synthesis and self-assembly. Macromolecules 2010, 43, 5530–5537. [Google Scholar] [CrossRef]
- Bielawski, C.W.; Grubbs, R.H. Living ring-opening metathesis polymerization. Progr. Polym. Sci. 2007, 32, 1–29. [Google Scholar] [CrossRef]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP toolbox upgraded. Polymer 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- Hardy, C.G.; Zhang, J.; Yan, Y.; Ren, L.; Tang, C. Metallopolymers with transition metals in the side-chain by living and controlled polymerization techniques. Progr. Polym. Sci. 2014, 39, 1742–1796. [Google Scholar] [CrossRef]
- Chen, Y.; Abdellatif, M.M.; Nomura, K. Olefin metathesis polymerization: Some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET). Tetrahedron 2018, 74, 619–643. [Google Scholar] [CrossRef]
- Hango, C.R.; Backlund, C.M.; Davis, H.C.; Posey, N.D.; Minter, L.M.; Tew, G.N. Non-covalent carrier hydrophobicity as a universal predictor of intracellular protein activity. Biomacromolecules 2021, 22, 2850–2863. [Google Scholar] [CrossRef]
- Kockelmann, J.; Stickdorn, J.; Kasmi, S.; De Vrieze, J.; Pieszka, M.; Ng, D.Y.W.; David, S.A.; De Geest, B.G.; Nuhn, L. Control over imidazoquinoline immune stimulation by pH-degradable poly(norbornene) nanogels. Biomacromolecules 2020, 21, 2246–2257. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.H.; Kim, H.-J.; Lee, D.-H.; Yang, S.K. Doubly dendronized poly(norbornene)s as siRNA delivery systems. Polymer 2021, 222, 123680. [Google Scholar] [CrossRef]
- Yersin, H. (Ed.) Highly Efficient OLEDs with Phosphorescent Materials; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-40594-7. [Google Scholar]
- Zysman-Colman, E. (Ed.) Iridium(III) in Optoelectronic and Photonics Applications; Wiley-VCH: Chichester, UK, 2017; ISBN 9781119007166. [Google Scholar]
- Yusoff, A.R.B.M.; Huckaba, A.J.; Nazeeruddin, M.K. Phosphorescent neutral iridium(III) complexes for organic light-emitting diodes. In Photoluminescent Materials and Electroluminescent Devices; Topics in Current Chemistry Collections; Springer: Cham, Switzerland, 2017; Volume 375, 39p. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(III) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef]
- Lo, K.K.-W. Luminescent rhenium(I) and iridium(III) polypyridine complexes as biological probes, imaging reagents, and photocytotoxic agents. Acc. Chem. Res. 2015, 48, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-N.; Li, G.; Leung, C.-H.; Ma, D.-L. Dual function luminescent transition metal complexes for cancer theranostics: The combination of diagnosis and therapy. Coord. Chem. Rev. 2019, 381, 79–103. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, J.; Chen, X. Cooperative photoredox catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, X.; Zou, L.; Zhao, M.; Liu, S.; Tao, P.; Jiang, J.; Zhao, Q.; Chen, Z.; Meng, X.; et al. A dual-emissive phosphorescent polymeric probe for exploring drug-induced liver injury via imaging of peroxynitrite elevation in vivo. ACS Appl. Mater. Interfaces 2020, 12, 12383–12394. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Finger, E.C.; Giaccia, A.J. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010, 29, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobita, S.; Yoshihara, T. Intracellular and in vivo oxygen sensing using phosphorescent iridium(III) complexes. Curr. Opin. Chem. Biol. 2016, 33, 39–45. [Google Scholar] [CrossRef]
- Yoshihara, T.; Hirakawa, Y.; Hosaka, M.; Nangaku, M.; Tobita, S. Oxygen imaging of living cells and tissues using luminescent molecular probes. J. Photochem. Photobiol. C Photochem. Rev. 2017, 30, 71–95. [Google Scholar] [CrossRef]
- Mizukami, K.; Katano, A.; Shiozaki, S.; Yoshihara, T.; Goda, N.; Tobita, S. In vivo O2 imaging in hepatic tissues by phosphorescence lifetime imaging microscopy using Ir(III) complexes as intracellular probes. Sci. Rep. 2020, 10, 21053. [Google Scholar] [CrossRef]
- Yasukagawa, M.; Shimada, A.; Shiozaki, S.; Tobita, S.; Yoshihara, T. Phosphorescent Ir(III) complexes conjugated with oligoarginine peptides serve as optical probes for in vivo microvascular imaging. Sci. Rep. 2021, 11, 4733. [Google Scholar] [CrossRef]
- Bochkarev, L.N.; Platonova, E.O.; Lermontova, S.A.; Klapshina, L.G.; Konev, A.N.; Abakumov, G.A. Iridium-containing polymers based on norbornene and 7-oxa-norbornene monomers: Synthesis and photophysical and biological properties. Polymer Sci. Ser. C 2019, 61, 58–64. [Google Scholar] [CrossRef]
- Bauer, T.; Slugovc, C. The thermo responsive behavior of glycol functionalized ring opening metathesis polymers. J. Polym. Sci. A Polym. Chem. 2010, 48, 2098–2108. [Google Scholar] [CrossRef]
- Platonova, E.O.; Pushkarev, A.P.; Ilichev, V.A.; Baranov, E.V.; Kovylina, T.A.; Bochkarev, L.N. Cyclometallated iridium(III) complex with 1-phenylisoquinoline and norbornene-substituted pyrazolonate ligands and related electroluminescent polymers. Russ. J. Coord. Chem. 2017, 43, 491–499. [Google Scholar] [CrossRef]
- Platonova, E.O.; Ilichev, V.A.; Bochkarev, L.N. Electroluminescent iridium-containing functionalized polynorbornenes emitting red light. Russ. J. Gen. Chem. 2018, 88, 985–991. [Google Scholar] [CrossRef]
- Sutthasupa, S.; Shiotsuki, M.; Matsuoka, H.; Masuda, T.; Sanda, F. Ring-opening metathesis block copolymerization of amino acid functionalized norbornene monomers. Effects of solvent and pH on micelle formation. Macromolecules 2010, 43, 1815–1822. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, Y.-G.; Shi, C.; Luo, J.; Zhu, X.X. Block and random copolymers bearing cholic acid and oligo(ethylene glycol) pendant groups: Aggregation, thermosensitivity, and drug loading. Biomacromolecules 2014, 15, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Sutthasupa, S.; Shiotsuki, M.; Masuda, T.; Sanda, F. Alternating ring-opening metathesis copolymerization of amino acid derived norbornene monomers carrying nonprotected carboxy and amino groups based on acid-base interaction. J. Am. Chem. Soc. 2009, 131, 10546–10551. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama, A.; Iwawaki, H.; Furugori, M.; Mukaide, T.; Kamatani, J.; Igawa, S.; Moriyama, T.; Miura, S.; Takiguchi, T.; Okada, S.; et al. Homoleptic cyclometalated iridium complexes with highly efficient red phosphorescence and application to organic light-emitting diode. J. Am. Chem. Soc. 2003, 125, 12971–12979. [Google Scholar] [CrossRef]
- Castor, K.J.; Metera, K.L.; Tefashe, U.M.; Serpell, C.J.; Mauzeroll, J.; Sleiman, H.F. Cyclometalated iridium(III) imidazole phenanthroline complexes as luminescent and electrochemiluminescent G-Quadruplex DNA binders. Inorg. Chem. 2015, 54, 6958–6967. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.K.; Mandal, S.; Purkayastha, P.; Gupta, P. Cyclometalated mono and dinuclear rhodium(III) and iridium(III) complexes with imidazolyl phenanthrolines: Synthesis and, photophysical and electrochemical characterization. Polyhedron 2015, 95, 14–23. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 0-8247-2377-5. [Google Scholar]
- Esipova, T.V.; Karagodov, A.; Miller, J.; Wilson, D.F.; Busch, T.M.; Vinogradov, S.A. Two new “protected” oxyphors for biological oximetry: Properties and application in tumor imaging. Anal. Chem. 2011, 83, 8756–8765. [Google Scholar] [CrossRef] [Green Version]
- Dunphy, I.; Vinogradov, S.A.; Wilson, D.F. Oxyphor R2 and G2: Phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal. Biochem. 2002, 310, 191–198. [Google Scholar] [CrossRef]
- Finikova, O.S.; Lebedev, A.Y.; Aprelev, A.; Troxler, T.; Gao, F.; Garnacho, C.; Muro, S.; Hochstrasser, R.M.; Vinogradov, S.A. Oxygen microscopy by two-photon-excited phosphorescence. ChemPhysChem 2008, 9, 1673–1679. [Google Scholar] [CrossRef] [Green Version]
- Sakadžić, S.; Roussakis, E.; Yaseen, M.A.; Mandeville, E.T.; Srinivasan, V.J.; Arai, K.; Ruvinskaya, S.; Devor, A.; Lo, E.H.; Vinogradov, S.A.; et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat. Methods 2010, 7, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babilas, P.; Liebsch, G.; Schacht, V.; Klimant, I.; Wolfbeis, O.S.; Szeimies, R.-M.; Abels, C. In Vivo Phosphorescence Imaging of pO2 Using Planar Oxygen Sensors. Microcirculation 2005, 12, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Babilas, P.; Schacht, V.; Liebsch, G.; Wolfbeis, O.S.; Landthaler, M.; Szeimies, R.-M.; Abels, C. Effects of light fractionation and different fluence rates on photodynamic therapy with 5-aminolaevulinic acid in vivo. Br. J. Cancer 2003, 88, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Allu, S.R.; Jiang, S.; Jia, M.; Gunn, J.R.; Yao, C.; LaRochelle, E.P.; Shell, J.R.; Bruza, P.; Gladstone, D.J.; et al. Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nat. Commun. 2020, 11, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukina, M.; Orlova, A.; Shirmanova, M.; Shirokov, D.; Pavlikov, A.; Neubauer, A.; Studier, H.; Becker, W.; Zagaynova, E.; Yoshihara, T.; et al. Interrogation of metabolic and oxygen states of tumors with fiber-based luminescence lifetime spectroscopy. Opt. Lett. 2017, 42, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Solomatina, A.I.; Su, S.-H.; Lukina, M.M.; Dudenkova, V.V.; Shcheslavskiy, V.I.; Wu, C.-H.; Chelushkin, P.S.; Chou, P.-T.; Koshevoy, I.O.; Tunik, S.P. Water-soluble cyclometalated platinum(II) and iridium(III) complexes: Synthesis, tuning of the photophysical properties, and in vitro and in vivo phosphorescence lifetime imaging. RSC Adv. 2018, 8, 17224–17236. [Google Scholar] [CrossRef] [Green Version]
- Wathier, M.; Stoddart, S.S.; Sheehy, M.J.; Grinstaff, M.W. Acidic polysaccharide mimics via ring-opening metathesis polymerization. J. Am. Chem. Soc. 2010, 132, 15887–15889. [Google Scholar] [CrossRef] [PubMed]
- Tezgel, A.Ö.; Telfer, J.C.; Tew, G.N. De novo designed protein transduction domain mimics from simple synthetic polymers. Biomacromolecules 2011, 12, 3078–3083. [Google Scholar] [CrossRef]
- Gueugnon, F.; Denis, I.; Pouliquen, D.; Collette, F.; Delatouche, R.; Héroguez, V.; Grégoire, M.; Bertrand, P.; Blanquart, C. Nanoparticles produced by ring-opening metathesis polymerization using norbornenyl-poly(ethylene oxide) as a ligand-free generic platform for highly selective in vivo tumor targeting. Biomacromolecules 2013, 14, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Wathier, M.; Lakin, B.A.; Bansal, P.N.; Stoddart, S.S.; Snyder, B.D.; Grinstaff, M.W. A large-molecular-weight polyanion, synthesized via ring-opening metathesis polymerization, as a lubricant for human articular cartilage. J. Am. Chem. Soc. 2013, 135, 4930–4933. [Google Scholar] [CrossRef] [PubMed]
- Denis, I.; El Bahhaj, F.; Collette, F.; Delatouche, R.; Gueugnon, F.; Pouliquen, D.; Pichavant, L.; Héroguez, V.; Grégoire, M.; Bertrand, P.; et al. Vorinostat-polymer conjugate nanoparticles for acid-responsive delivery and passive tumor targeting. Biomacromolecules 2014, 15, 4534–4543. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-G.; Zhu, X.X. Thermo- and pH-responsive copolymers bearing cholic acid and oligo(ethylene glycol) pendants: Self-assembly and pH-controlled release. ACS Appl. Mater. Interfaces 2015, 7, 24649–24655. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Xie, N.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Modular design of poly(norbornenes) for organelle-specific imaging in tumor cells. Biomacromolecules 2016, 17, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Patra, D.; Dinda, H.; Chakraborty, I.; Shashank, L.; Bhattacharyya, R.; Sarma, J.D.; Shunmugam, R. Super paramagnetic norbornene copolymer functionalized with biotin and doxorubicin: A potential unique site-specific theranostic agent. Macromolecules 2016, 49, 2411–2418. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dinda, H.; Chakraborty, I.; Bhattacharyya, R.; Sarma, J.D.; Shunmugam, R. Engineering camptothecin-derived norbornene polymers for theranostic application. ACS Omega 2017, 2, 2848–2857. [Google Scholar] [CrossRef] [Green Version]
- Xie, N.; Feng, K.; Shao, J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Luminescence-tunable polynorbornenes for simultaneous multicolor imaging in subcellular organelles. Biomacromolecules 2018, 19, 2750–2758. [Google Scholar] [CrossRef]
- Papkovsky, D.B.; Dmitriev, R.I. Imaging of oxygen and hypoxia in cell and tissue samples. Cell. Mol. Life Sci. 2018, 75, 2963–2980. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Stolwijk, J.A.; Sperber, M.; Meier, R.J.; Wegener, J.; Wolfbeis, O.S. Ultra-small, highly stable, and membrane-impermeable fluorescent nanosensors for oxygen. Methods Appl. Fluoresc. 2013, 1, 035002. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, S.; Jia, M.; Liu, Y.; Shang, J.; Guo, Y.; Xu, J.; Wu, D. Hypoxia-sensitive bis(2-(2′-benzothienyl)pyridinato-N,C3′)iridium[poly(n-butyl cyanoacrylate]/chitosan nanoparticles and their phosphorescence tumor imaging in vitro and in vivo. Nanoscale 2013, 5, 12633–12644. [Google Scholar] [CrossRef] [PubMed]
- Kondrashina, A.V.; Dmitriev, R.I.; Borisov, S.M.; Klimant, I.; O’Brien, I.; Nolan, Y.M.; Zhdanov, A.V.; Papkovsky, D.B. A phosphorescent nanoparticle-based probe for sensing and imaging of (intra)cellular oxygen in multiple detection modalities. Adv. Funct. Mater. 2012, 22, 4931–4939. [Google Scholar] [CrossRef]
- Yip, A.M.H.; Lo, K.K.W. Luminescent rhenium(I), ruthenium(II), and iridium(III) polypyridine complexes containing a poly(ethylene glycol) pendant or bioorthogonal reaction group as biological probes and photocytotoxic agents. Coord. Chem. Rev. 2018, 361, 138–163. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Borisov, S.M.; Düssmann, H.; Sun, S.; Müller, B.J.; Prehn, J.; Baklaushev, V.P.; Klimant, I.; Papkovsky, D.B. Versatile conjugated polymer nanoparticles for high-resolution O2 imaging in cells and 3D tissue models. ACS Nano 2015, 9, 5275–5288. [Google Scholar] [CrossRef]
- López Arbeloa, F.; Ruiz Ojeda, P.; López Arbeloa, I. Fluorescence self-quenching of the molecular forms of rhodamine B in aqueous and ethanolic solutions. J. Lumin. 1989, 44, 105–112. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. The Measurement of photoluminescence quantum yields. A review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Shcheslavskiy, V.I.; Neubauer, A.; Bukowiecki, R.; Dinter, F.; Becker, W. Combined fluorescence and phosphorescence lifetime imaging. Appl. Phys. Lett. 2016, 108, 091111. [Google Scholar] [CrossRef]
- Shcheslavskiy, V.I.; Shirmanova, M.V.; Dudenkova, V.V.; Lukyanov, K.A.; Gavrina, A.I.; Shumilova, A.V.; Zagaynova, E.V.; Becker, W. Fluorescence time-resolved macroimaging. Opt. Lett. 2018, 43, 3152–3155. [Google Scholar] [CrossRef]
- Hersey, J.S.; Meller, A.; Grinstaff, M.W. Functionalized nanofiber meshes enhance immunosorbent assays. Anal. Chem. 2015, 87, 11863–11870. [Google Scholar] [CrossRef] [Green Version]
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands. Org. Lett. 1999, 1, 953–956. [Google Scholar] [CrossRef]
- Love, J.A.; Morgan, J.P.; Trnka, T.M.; Grubbs, R.H. A practical and highly active ruthenium-based catalyst that effects the cross metathesis of acrylonitrile. Angew. Chem. Int. Ed. 2002, 41, 4035–4037. [Google Scholar] [CrossRef]










| Polymer | λmaxabs/nm (log ε) in CH2Cl2 | λmaxem/nm (CH2Cl2) | Quantum Yield, % (in CH2Cl2) | Quantum Yield, % (in Water) | Chromaticity Coordinates in the CIE Diagram (x; y) | ||
|---|---|---|---|---|---|---|---|
| (a) | (b) | (a) | (b) | ||||
| P1 | 297 sh (4.29), 344 (3.97), 476 (3.60), 550 (3.26) | 618 | 10.0 | 2.7 | 3.8 | 2.8 | 0.66; 0.34 |
| P2 | 300 sh (4.45), 353 (4.29), 472 (3.91), 525 (3.90) | 657 | 2.5 | 0.5 | 0.5 | 0.3 | 0.71; 0.29 |
| P3 | 299 sh (4.08), 346 (3.75), 477 (2.95), 552 (2.59) | 618 | 9.3 | 3.6 | 2.7 | 2.0 | 0.66; 0.34 |
| P4 | 302 sh (4.18), 353 (4.04), 471 (3.66), 527 (3.65) | 657 | 2.6 | 0.5 | 0.4 | 0.2 | 0.71; 0.29 |
| P1 | P3 | |
|---|---|---|
| τ0, μs | 1.5 ± 0.07 | 1.5 ± 0.08 |
| τ, μs (τ in BSA, μs) | 1.1 ± 0.06 (1.2 ± 0.07) | 1.3 ± 0.06 (1.2 ± 0.06) |
| Dynamic range r = τ0/τ | 1.36 ± 0.072 | 1.15 ± 0.07 |
| Photon count rate (for τ) | 5890 | 5600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochkarev, L.N.; Parshina, Y.P.; Gracheva, Y.V.; Kovylina, T.A.; Lermontova, S.A.; Klapshina, L.G.; Konev, A.N.; Lopatin, M.A.; Lukina, M.M.; Komarova, A.D.; et al. Red Light-Emitting Water-Soluble Luminescent Iridium-Containing Polynorbornenes: Synthesis, Characterization and Oxygen Sensing Properties in Biological Tissues In Vivo. Molecules 2021, 26, 6349. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216349
Bochkarev LN, Parshina YP, Gracheva YV, Kovylina TA, Lermontova SA, Klapshina LG, Konev AN, Lopatin MA, Lukina MM, Komarova AD, et al. Red Light-Emitting Water-Soluble Luminescent Iridium-Containing Polynorbornenes: Synthesis, Characterization and Oxygen Sensing Properties in Biological Tissues In Vivo. Molecules. 2021; 26(21):6349. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216349
Chicago/Turabian StyleBochkarev, Leonid N., Yulia P. Parshina, Yana V. Gracheva, Tatyana A. Kovylina, Svetlana A. Lermontova, Larisa G. Klapshina, Aleksey N. Konev, Mikhail A. Lopatin, Maria M. Lukina, Anastasia D. Komarova, and et al. 2021. "Red Light-Emitting Water-Soluble Luminescent Iridium-Containing Polynorbornenes: Synthesis, Characterization and Oxygen Sensing Properties in Biological Tissues In Vivo" Molecules 26, no. 21: 6349. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26216349