Synthesis and Structure of Nido-Carboranyl Azide and Its “Click” Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 9-N3(CH2)3Me2N-nido-7,8-C2B9H11 and Its “Click” Reaction with Phenylacetylene
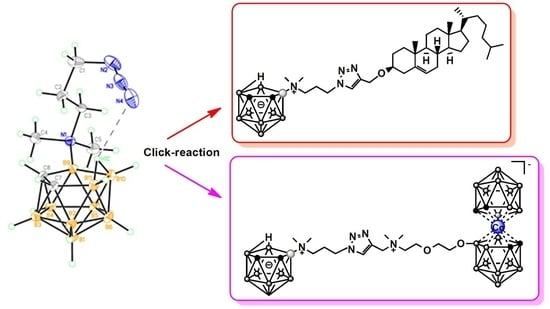
2.2. Synthesis of Nido-Carboranyl Cholesterol Derivative with Charge-Compensated Group
2.3. Synthesis of Zwitter-Ionic Boron-Enriched Cluster Compounds Bearing a 1,2,3-Triazol-Metallacarborane-Nido-Carborane Conjugated Systems
2.4. Single-Crystal X-ray Diffraction Studies
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of 9-N3(CH2)3Me2N-nido-7,8-C2B9H11 2
General Procedure for the Synthesis of the Compounds 3, 5, 10–12
3.3. Synthesis of 9-(Ph)C-CH-N3(CH2)3Me2N-nido-7,8-C2B9H11 3
3.4. Synthesis of 9-3β-Chol-O(CH2)C-CH-N3(CH2)3Me2N-nido-7,8-C2B9H11 5
3.5. Synthesis of 9-[(8′-Me2N(CH2)3N3CCH(CH2)NMe2(CH2CH2O)2-1′,2′-C2B9H10)-3′,3″-Co(1″,2″-C2B9H11)]-nido-7,8-C2B9H11 10
3.6. Synthesis of 9-[(8′-Me2N(CH2)3N3CCH(CH2)NMe2(CH2)5-1′,2′-C2B9H10)-3′,3″-Co(1″,2″-C2B9H11)]-nido-7,8-C2B9H11 11
3.7. Synthesis of 9-[(8′-Me2N(CH2)3N3CCH(CH2)NMe2(CH2)5-1′,2′-C2B9H10)-3′,3″-Fe(1″,2″-C2B9H11)]-nido-7,8-C2B9H11 12
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016; 905p, ISBN 978-0-12-801894-1. [Google Scholar]
- Hosmane, N.S.; Maguire, J.A. Comprehensive Organometallic Chemistry III.; Michael, D., Mingos, P., Crabtree, R.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; 264p, ISBN 978-0-0804-4601-1. [Google Scholar]
- Hawthorne, M.F. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew. Chem. Int. Ed. Engl. 1993, 32, 950–998. [Google Scholar] [CrossRef]
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. The medicinal chemistry of carboranes. Coord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Valliant, J.F. The bioinorganic and medicinal chemistry of carboranes: From new drug discovery to molecular imaging and therapy. Dalton Trans. 2007, 4240–4251. [Google Scholar] [CrossRef] [PubMed]
- Řezačova, P.; Cigler, P.; Matejiček, P.; Lipšik, M.; Pokorna, J.; Grüner, B.; Konvalinka, J. Medicinal application of carboranes: Inhibition of HIV protease. Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; 70p, ISBN 978-1-43-982662-1. [Google Scholar]
- Tolmachev, V.V.; Sjöberg, S. Polyhedral boron compounds as potential linkers for attachment of radiohalogens to targeting proteins and peptides. A review. Collect. Czech. Chem. Commun. 2002, 67, 913–935. [Google Scholar] [CrossRef]
- Lesnikowski, Z.J. New Opportunities in Boron Chemistry for Medical Applications. Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; 3p, ISBN 978-1-43-982662-1. [Google Scholar]
- Sivaev, I.B.; Bregadze, V.I. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 11, 1433–1450. [Google Scholar] [CrossRef]
- Tsygankova, A.R.; Kanygin, V.V.; Kasatova, A.I.; Zavyalov, E.L.; Guselnikova, T.Y.; Kichigin, A.I.; Mukhamadiyarov, R.A. Determination of boron by inductively coupled plasma atomic emission spectroscopy. Biodistribution of 10B in tumor-bearing mice. Russ. Chem. Bull. 2020, 69, 601–607. [Google Scholar] [CrossRef]
- Stogniy, M.; Abramova, E.N.; Lobanova, I.A.; Sivaev, I.B.; Bragin, V.I.; Petrovskii, P.V.; Tsupreva, V.N.; Sorokina, O.V.; Bregadze, V.I. Synthesis of Functional Derivatives of 7,8-Dicarba-nido-undecaborate Anion by Ring-Opening of Its Cyclic Oxonium Derivatives. Collect. Czechoslov. Chem. Commun. 2007, 72, 1676–1688. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Kazakov, G.S.; Sivaev, I.B.; Bregadze, V.I. Synthesis of podands with nido-carboranyl groups as a basis for construction of crown ethers with an incorporated metallacarborane moiety. Russ. Chem. Bull. 2013, 62, 699–704. [Google Scholar] [CrossRef]
- Kazakov, G.S.; Stogniy, M.Y.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Kirilin, A.D.; Bregadze, V.I. Synthesis of crown ethers with the incorporated cobalt bis(dicarbollide) fragment. J. Organomet. Chem. 2015, 798, 196–203. [Google Scholar] [CrossRef]
- Wojtczak, B.A.; Andrysiak, A.; Grüner, B.; Lesnikowski, Z.J. “Chemical ligation”: A versatile method for nucleoside modification with boron clusters. Chem. Eur. J. 2008, 14, 10675–10682. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Sivaev, I.B.; Anufriev, S.A.; Timofeev, S.V.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. A new approach to the synthesis of functional derivatives of nido-carborane: Alkylation of [9-MeS-nido-7,8-C2B9H11]". Dalton Trans. 2014, 43, 5044–5053. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B–H···π Interactions between the B–H–B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 38–39, 4436–4443. [Google Scholar] [CrossRef] [Green Version]
- Anufriev, S.A.; Zakharova, M.V.; Sivaev, I.B.; Bregadze, V.I. New carborane-containing acids and amines. Russ. Chem. Bull. 2017, 66, 1643–1649. [Google Scholar] [CrossRef]
- Erokhina, S.A.; Stogniy, M.Y.; Suponitsky, K.Y.; Kosenko, I.D.; Sivaev, I.B.; Bregadze, V.I. Experimental crystal structure determination. Polyhedron. 2018, 153, 145–151. [Google Scholar] [CrossRef]
- Timofeev, S.V.; Zhidkova, O.B.; Prikaznova, E.A.; Sivaev, I.B.; Semioshkin, A.; Godovikov, I.A.; Starikova, Z.A.; Bregadze, V.I. Direct synthesis of nido-carborane derivatives with pendant functional groups by copper-promoted reactions with dimethylalkylamines. J. Organomet. Chem. 2014, 757, 21–27. [Google Scholar] [CrossRef]
- Stogniy, M.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Sivaev, I.B.; Bregadze, V.I. Nucleophilic addition reactions to the ethylnitrilium derivative of nido-carborane 10-EtCN-7,8-C2B9H11. New J. Chem. 2018, 42, 17958–17967. [Google Scholar] [CrossRef]
- Stanislav, I.; Presolski, V.; Hong, P.; Finn, M.G. Copper-catalyzed azide-alkyne click chemistry for bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Jiang, Z.; He, H.; Liu, H.; Thayumanavan, S. Azide-Terminated RAFT Polymers for Biological Applications. Curr. Protoc. Chem. Biol. 2020, 12, e85. [Google Scholar] [CrossRef]
- Meldal, M.; Diness, F. Recent Fascinating Aspects of the CuAAC Click Reaction. Trends Chemistry. 2020. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Chen, X. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef] [PubMed]
- Yet, L. Five-Membered Ring Systems: With More than One N Atom. Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; 229p, ISBN 978-0-08-044005-7. [Google Scholar]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700–111737. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Karypidou, K.; Ribone, S.R.; Quevedo, M.A.; Persoons, L.; Pannecouque, C.; Helsen, C.; Claessens, F.; Dehaen, W. Synthesis, biological evaluation and molecular modeling of a novel series of fused 1,2,3-triazoles as potential anti-coronavirus agents. Bioorg. Med. Chem. Lett. 2018, 28, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Comprehensive review on the anti-bacterial activity of 1,2,3-triazole hybrids. Eur. J. Med. Chem. 2019, 168, 357–372. [Google Scholar] [CrossRef] [PubMed]
- El Malah, T.; Nour, H.F.; Satti, A.A.E.; Hemdan, B.A.; ElSayed, W.A. Design, synthesis and antimicrobial activities of 1,2,3- triazole glycoside clickamers. Molecules 2020, 25, 790. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, B.; Kumar, P.V.; Reddy, P.N.; Venu, S.; Shyam, P.; Krupadanam, G.L.D. Design, synthesis, antioxidant and antibacterial activities of novel 2-((1-benzyl-1h-1,2,3-triazol-4- yl)methyl)-5-(2hchromen- 3-yl)-2h-tetrazoles. Russ. J. Bioorg. Chem. 2018, 44, 244–251. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Luxmi, R. Synthesis, antibacterial and antioxidant activities of naphthyl-linked disubstituted 1,2,3-triazoles. J. Heterocycl. Chem. 2020, 57, 2400–2409. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Semioshkin, A.A.; Las’Kova, J.N.; Berzina, M.Y.; Lobanova, I.A.; Sivaev, I.B.; Grin, M.A.; Titeev, R.A.; Brittal, D.I.; Ulybina, O.V.; et al. Novel types of boronated chlorine6conjugates via ‘click chemistry’. Appl. Organomet. Chem. 2009, 23, 370–374. [Google Scholar] [CrossRef]
- Best, M.D. “Click” chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry 2009, 48, 6571–6584. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Druzina, A.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel carboranyl azides and “click” reactions thereof. J. Organomet. Chem. 2019, 904, 121007–121014. [Google Scholar] [CrossRef]
- Semioshkin, A.; Laskova, J.; Wojtczak, B.; Andrysiak, A.; Godovikov, I.; Bregadze, V.; Lesnikowski, Z.J. Synthesis of closo-dodecaborate based nucleoside conjugates. J. Organomet. Chem. 2009, 694, 1375–1379. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Osipov, S.N.; Grebenyuk, J.N.; Nizhnik, E.A.; Godovikov, I.A.; Shchetnikov, G.T.; Bregadze, V.I. An effective approach to 1,2,3-triazole-containing 12-vertex closo-dodecaborates. Collect. Czech. Chem. Commun. 2007, 72, 1717–1724. [Google Scholar] [CrossRef]
- Kahl, S.B.; Pate, D.; Laster, B.H.; Popenoe, E.A.; Fairchild, R.G. In Vitro Biological Efficacy of Boronated Low Density Lipoproteins for NCT. Progress in Neutron Capture Therapy for Cancer; Allen, B.J., Moore, D.E., Harrington, B.V., Eds.; Plenum Press: New York, NY, USA, 1992; 365p, ISBN 978-1-4615-3384-9. [Google Scholar]
- Feakes, D.A.; Spinler, J.K.; Harris, F.R. Synthesis of boron-containing cholesterol derivatives for incorporation into unilamellar liposomes and evaluation as potential agents for BNCT. Tetrahedron 1999, 55, 11177–11186. [Google Scholar] [CrossRef]
- Ji, B.; Peacock, G.; Lu, D.R. Synthesis of cholesterol-carborane conjugate for targeted drug delivery. Bioorgan. Med. Chem. Lett. 2002, 12, 2455–2458. [Google Scholar] [CrossRef]
- Barth, R.F. Boron neutron capture therapy at the crossroads: Challenges and opportunities. Appl. Radiat. Isot. 2009, 67, S3–S6. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug. Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Eloy, J.O.; Claro de Souza, M.; Petrilli, R.; Abriata Barcellos, J.P.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids. Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. Advances in tumor targeted liposomes. Curr. Mol. Med. 2018, 18, 44–57. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Andreichuk, E.P.; Zhidkova, O.B.; Kosenko, I.D.; Semioshkin, A.; Sivaev, I.B.; Mandal, S.; Shen, Z.; Bregadze, V. ‘Click’ synthesis of cobalt bis(dicarbollide)–cholesterol conjugates. Mendeleev Commun. 2019, 29, 628–630. [Google Scholar] [CrossRef]
- Druzina, A.A.; Kosenko, I.D.; Zhidkova, O.B.; Ananyev, I.V.; Timofeev, S.V.; Bregadze, V.I. Novel cobalt bis(dicarbollide) based on terminal alkynes and their “click” reactions. Eur. J. Inorg. Chem. 2020, 27, 2658–2665. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Kosenko, I.D. Synthesis of conjugates of closo-dodecaborate dianion with cholesterol using a “click” reaction. Russ. Chem. Bull. 2020, 69, 1080–1084. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Sivaev, I.B.; Semioshkin, A.; Shmal’ko, A.V.; Kosenko, I.D.; Lebedeva, K.V.; Mandal, S.; Sreejyothi, P.; Dubey, R.D.; Sarkar, A.; et al. Boron-containing lipids and liposomes: New conjugates of cholesterol with polyhedral boron hydrides. Chem. Eur. J. 2020, 26, 13832–13841. [Google Scholar] [CrossRef]
- Dubey, R.D.; Sarkar, A.; Shen, Z.; Bregadze, V.I.; Sivaev, I.B.; Druzina, A.A.; Zhidkova, O.B.; Shmal’ko, A.V.; Kosenko, I.D.; Sreejyothi, P.; et al. Effects of linkers on the development of liposomal formulation of cholesterol conjugated cobalt bis(dicarbollides). J. Pharm. Sci. 2020. [Google Scholar] [CrossRef]
- Lee, W.; Sarkar, S.; Ahn, H.; Kim, J.Y.; Lee, Y.J.; Chang, Y.; Yoo, J. PEGylated liposome encapsulating nido-carborane showed significant tumor suppression in boron neutron capture therapy (BNCT). Biochem. Biophys. Res. Commun. 2020, 522, 669–675. [Google Scholar] [CrossRef]
- Aly, M.R.E.; Saad, H.A.; Mohamed, M.A.N. Difluoromethyl ketones: Potent inhibitors of wild type and carbamate-insensitive G119S mutant anopheles gambiae acetylcholinesterase. Bioorg. Med. Chem. Lett. 2015, 25, 4405–4411. [Google Scholar] [CrossRef] [Green Version]
- Šícha, V.; Farràs, P.; Štíbr, B.; Teixidor, F.; Grüner, B.; Viñas, C. Syntheses of C-substituted icosahedral dicarbaboranes bearing the 8-dioxane-cobalt bisdicarbollide moiety. J. Organomet. Chem. 2009, 694, 1599–1601. [Google Scholar] [CrossRef]
- Mishima, Y.; Ichihashi, M.; Hatta, S.; Honda, C.; Yamamura, K.; Nakagawa, T. New thermal neutron capture therapy for malignant melanoma: Melanogenesis-seeking 10B molecule-melanoma cell interaction from in vitro to first clinical trial. Pigm. Cell Res. 1989, 2, 226–234. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents forb neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, I.; Garcia-Mendiola, T.; Sato, S.; Pita, M.; Nakamura, H.; Lorenzo, E.; Teixidor, F.; Marques, F.; Vinas, C. Metallacarboranes on the road to anticancer therapies: Cellular uptake, DNA interaction, and biological evaluation of cobaltabisdicarbollide [COSAN]−. Chem. Eur. J. 2018, 24, 17239–17254. [Google Scholar] [CrossRef] [PubMed]
- Tarrés, M.; Canetta, E.; Paul, E.; Forbes, J.; Azzouni, K.; Viñas, C.; Teixidor, F.; Harwood, A. Biological interaction of living cells with COSAN-based synthetic vesicles. J. Sci. Rep. 2015, 5, 7804–7811. [Google Scholar] [CrossRef] [PubMed]
- Bauduin, P.; Prevost, S.; Farràs, P.; Teixidor, F.; Diat, O.; Zemb, T. A theta-shaped amphiphilic cobaltabisdicarbollide anion: Transition from monolayer vesicles to micelles. Angew. Chem. Int. Ed. 2011, 50, 5298–5300. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, D.C.; Viñas, C.; Teixidor, F.; Faraudo, J. Atomistic simulations of COSAN: Amphiphiles without a head-and-tail design display «head and tail» surfactant behavior. J. Angew. Chem. Int. Ed. 2020, 59, 3088–3092. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Mccrate, J.M.; Li, H.; Lee, J.C.-M. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708–105717. [Google Scholar] [CrossRef] [Green Version]
- Tatur, S.; MacCarini, M.; Barker, R.; Nelson, A.; Fragneto, G. Effect of functionalized gold nanoparticles on floating lipid bilayers. Langmuir 2013, 29, 6606–6614. [Google Scholar] [CrossRef]
- Lobanova, I.; Kosenko, I.; Laskova, J.; Ananyev, I.; Druzina, A.; Godovikov, I.; Bregadze, V.; Qi, S.; Lesnikowski, Z.J.; Semioshkin, A. Synthesis and the structure of 8-tetrahydrofuronium and 8-tetrahydropyronium derivatives of iron bis(dicarbollide)(-I) and their cleavage reactions. Dalton Trans. 2015, 44, 1571–1584. [Google Scholar] [CrossRef]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules: From Solid State to DNA, Drug Design; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2007; 567p, ISBN 978-3-527-30748-7. [Google Scholar]
- Pendas, A.M.; Francisco, E.; Blanco, M.A.; Gatti, C. Bond paths as privileged exchange channels. Chem. Eur. J. 2007, 13, 9362–9371. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Ananyev, I.V. Interatomic exchange-correlation interaction energy from a measure of quantum theory of atoms in molecules topological bonding: A diatomic case. J. Comput. Chem. 2020, 41, 2213–2222. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing non-covalent iinteractions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanova, A.; Lyssenko, K.; Ananyev, I. Estimations of energy of noncovalent bonding from integrals over interatomic zero-flux surfaces: Correlation trends and beyond. J. Comput. Chem. 2018, 39, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll, version 13.05.06; TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druzina, A.A.; Zhidkova, O.B.; Dudarova, N.V.; Kosenko, I.D.; Ananyev, I.V.; Timofeev, S.V.; Bregadze, V.I. Synthesis and Structure of Nido-Carboranyl Azide and Its “Click” Reactions. Molecules 2021, 26, 530. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030530
Druzina AA, Zhidkova OB, Dudarova NV, Kosenko ID, Ananyev IV, Timofeev SV, Bregadze VI. Synthesis and Structure of Nido-Carboranyl Azide and Its “Click” Reactions. Molecules. 2021; 26(3):530. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030530
Chicago/Turabian StyleDruzina, Anna A., Olga B. Zhidkova, Nadezhda V. Dudarova, Irina D. Kosenko, Ivan V. Ananyev, Sergey V. Timofeev, and Vladimir I. Bregadze. 2021. "Synthesis and Structure of Nido-Carboranyl Azide and Its “Click” Reactions" Molecules 26, no. 3: 530. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26030530