Polymerization Isomerism in Co-M (M = Cu, Ag, Au) Carbonyl Clusters: Synthesis, Structures and Computational Investigation
Abstract
:1. Introduction
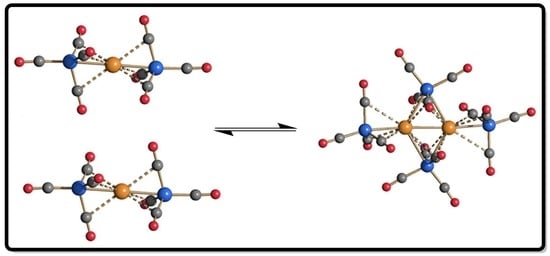
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthesis of [PPN][Cu{Co(CO)4}2] ([PPN](2))
3.3. Synthesis of [NEt4][Cu{Co(CO)4}2] ([NEt4](2))
3.4. Synthesis of [NEt4][Ag{Co(CO)4}2] ([NEt4](3))
3.5. Synthesis of [PPN][Au{Co(CO)4}2] ([PPN](4))
3.6. Synthesis of [NEt4][Au{Co(CO)4}2] ([NEt4](4))
3.7. Synthesis of Na2[Ag2{Co(CO)4}4]·C4H6O2 (Na2(5)·C4H6O2)
3.8. Synthesis of [PPN]2[Ag2{Co(CO)4}4]·C5H12 ([PPN]2(5)·C5H12)
3.9. Synthesis of [NBu4]2[Ag2{Co(CO)4}4] ([NBu4]2(5))
3.10. Synthesis of [NMe4]2[Ag2{Co(CO)4}4] ([NMe4]2(5))
3.11. Synthesis of Na2[Au{Co3(CO)9}2][Au{Co2(CO)7}2]·6H2O (Na2(7)(6)·6H2O)
3.12. Synthesis of [NMe3(CH2Ph)]2[Co6(CO)15] ([NMe3(CH2Ph)]2(8))
3.13. Synthesis of [PPN]2[Co(THF)4(BF4)2][BF4]2·4CH2Cl2 ([PPN]2(9)[BF4]2·4CH2Cl2)
3.14. X-ray Crystallographic Study
3.15. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, W.W.; Zeng, X.C.; Gao, Y. The structural isomerism in gold nanoclusters. Nanoscale 2018, 10, 9476–9483. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Li, Y.-Z.; Li, M.-B.; Yuan, J.; Yang, J.; Wu, Z.; Jin, R. Structural Isomerism in Gold Nanoparticles Revealed by X-ray Crystallography. Nat. Commun. 2015, 6, 8667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhou, M.; Li, Q.; Gronlund, H.; Jin, R. Isomerization-induced enhancement of luminescence in Au28(SR)20nanoclusters. Chem. Sci. 2020, 11, 8176–8183. [Google Scholar] [CrossRef]
- He, L.; He, X.; Wang, J.; Qu, Y.; Su, X.; Zheng, J.; Zhao, X. The positional isomerism in bimetal nanoclusters. CrystEngComm 2020, 22, 6975–6978. [Google Scholar] [CrossRef]
- Guan, Z.-J.; Hu, F.; Li, J.-J.; Wen, Z.-R.; Lin, Y.-M.; Wang, Q.-M. Isomerization in Alkynyl-Protected Gold Nanoclusters. J. Am. Chem. Soc. 2020, 142, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Jadzinsky, P.D.; Calero, G.; Ackerson, D.A.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 A Resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y.; Zhu, M.; Jin, R. Atomically precise alloy nanoclusters: Syntheses, structures, and properties. Chem. Soc. Rev. 2020, 49, 6443–6514. [Google Scholar] [CrossRef]
- Du, X.; Jin, R. Atomic-precision engineering of metal nanoclusters. Dalton Trans. 2020, 49, 10701–10707. [Google Scholar] [CrossRef]
- Li, Y.; Higaki, T.; Du, X.; Jin, R. Chirality and Surface Bonding Correlation in Atomically Precise Metal Nanoclusters. Adv. Mater. 2020, 32, 1905488. [Google Scholar] [CrossRef]
- Jin, S.; Xu, F.; Du, W.; Kang, X.; Chen, S.; Zhang, J.; Li, X.; Hu, D.; Wang, S.; Zhu, M. Isomerism in Au-Ag Alloy Nanoclusters: Structure Determination and Enantioseparation of [Au9Ag12(SR)4(dppm)6X6]3+. Inorg. Chem. 2018, 57, 5114–5119. [Google Scholar] [CrossRef]
- Zhuang, S.; Liao, L.; Yuan, J.; Xia, N.; Zhao, Y.; Wang, C.; Gan, Z.; Yan, N.; He, L.; Li, J.; et al. Fcc versus Non-fcc Structural Isomerism of Gold Nanoparticles with Kernel Atom Packing Dependent Photoluminescence. Angew. Chem. Int. Ed. 2019, 58, 4510–4514. [Google Scholar] [CrossRef]
- Zacchini, S. Using Metal Carbonyl Clusters to Develop a Molecular Approach towards Metal Nanoparticles. Eur. J. Inorg. Chem. 2011, 2011, 4125–4145. [Google Scholar] [CrossRef]
- Bender, R.; Braunstein, P.; Tiripicchio, A.; Tiripicchio-Camellini, M. Skeletal Isomerization of the [Pt3(μ-PPh2)3Ph(PPh3)2] Cluster by Recrystallization in Various Solvents. Angew. Chem. Int. Ed. 1985, 24, 861–862. [Google Scholar] [CrossRef]
- Bender, R.; Braunstein, P.; Dedieu, A.; Ellis, P.D.; Huggins, B.; Harvey, P.D.; Sappa, E.; Tiripicchio, A. Synthetic, Structural, Spectroscopic and Theoretical Studies of Structural Isomerism of the Cluster [Pt3(μ-PPh2)3Ph(PPh3)2]. A Unique Example of Core Isomerism in Phosphine Phosphido-Rich Clusters. Inorg. Chem. 1996, 35, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Welter, R.; Braunstein, P. Phosphanido-bridged triangular platinum clusters as versatile platforms: A personal account. Inorg. Chim. Acta 2015, 424, 20–28. [Google Scholar] [CrossRef]
- Ciabatti, I.; Femoni, C.; Iapalucci, M.C.; Longoni, G.; Lovato, T.; Zacchini, S. PPh3-Derivatives of [Pt3n(CO)6n]2– (n = 2–6) Chini’s Clusters: Syntheses, Structures, and 31P NMR Studies. Inorg. Chem. 2013, 52, 4384–4395. [Google Scholar] [CrossRef] [PubMed]
- Ciabatti, I.; Femoni, C.; Iapalucci, M.C.; Longoni, G.; Zacchini, S. Platinum Carbonyl Clusters Chemistry: Four Decades of Challenging Nanoscience. J. Clust. Sci. 2014, 25, 115–146. [Google Scholar] [CrossRef]
- Berti, B.; Bortoluzzi, M.; Ceriotti, A.; Cesari, C.; Femoni, C.; Iapalucci, M.C.; Zacchini, S. Further insights into platinumcarbonyl Chini clusters. Inorg. Chim. Acta 2020, 512, 119904. [Google Scholar] [CrossRef]
- Fehlner, T.P.; Housecroft, C.E.; Wade, K. Electron Counting in Cluster Species. The Significance of the Structure and Isomerism of the Iron-Molybdenum-Sulfur Cluster Compound (Dicyclopentadienyldimolybdenum)diironOcatacarbonyl Disulfide, (η5-C5H5)2Mo2Fe2(CO)8S2, a Hexanuclear Species in Which Two Mo2FeS Tetrahedra Share a Common Mo-Mo Edge. Organometallcs 1983, 2, 1426–1428. [Google Scholar]
- Berti, B.; Bortoluzzi, M.; Cesari, C.; Femoni, C.; Iapalucci, M.C.; Mazzoni, R.; Vacca, F.; Zacchini, S. Thermal Growth of Au-Fe Heterometallic Carbonyl Clusters Containing N-Heterocyclic Carbene and Phosphine Ligands. Inorg. Chem. 2020, 59, 2228–2240. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Morshedi, M.; Moxey, G.J.; Barlow, A.; Cifuentes, M.P.; Humphrey, M.G. Dynamic Permutational Isomerism in a closo-cluster. Chem. Eur. J. 2016, 22, 5128–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciabatti, I.; Femoni, C.; Iapalucci, M.C.; Ienco, A.; Longoni, G.; Manca, G.; Zacchini, S. Intramolecular d10-d10Interactions in a Ni6C(CO)9(AuPPh3)4 Bimetallic Nickel-Gold Carbide Carbonyl Cluster. Inorg. Chem. 2013, 52, 10559–10565. [Google Scholar] [CrossRef] [PubMed]
- Ciabatti, I.; Femoni, C.; Hayatifar, M.; Iapalucci, M.C.; Zacchini, S. Co5C and Co4C carbido carbonyl clusters stabilized by [AuPPh3]+ fragments. Inorg. Chim. Acta 2015, 428, 203–211. [Google Scholar] [CrossRef]
- Ciabatti, I.; Femoni, C.; Hayatifar, M.; Iapalucci, M.C.; Ienco, A.; Longoni, G.; Manca, G.; Zacchini, S. Octahedral Co-Carbide Carbonyl Clusters Decorated by [AuPPh3]+ Fragments: Synthesis, Structural Isomerism, and Aurophilic Interactions of Co6C(CO)12(AuPPh3)4. Inorg. Chem. 2014, 53, 9761–9770. [Google Scholar] [CrossRef]
- Femoni, C.; Iapalucci, M.C.; Longoni, G.; Zacchini, S.; Fedi, S.; De Biani, F.F. Nickel poly-acetylide carbonyl clusters: Structural features, bonding and electrochemical behavior. Dalton Trans. 2012, 41, 4649–4663. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.F.; Johnson, B.F.G.; Lewis, J.; McPartlin, M.; Nelson, W.J.H. [H3Ru4(CO)12]–: The X-ray crystallographic determination of two structural isomers. J. Chem. Soc. Chem. Commun. 1978, 920–921. [Google Scholar] [CrossRef]
- Cesari, C.; Ciabatti, I.; Femoni, C.; Iapalucci, M.C.; Mancini, F.; Zacchini, S. HeterolepticChini-Type Platinum Clusters: Synthesis and Characterization of Bis-Phosphine Derivatives of [Pt3n(CO)6n]2– (n = 2–4). Inorg. Chem. 2017, 56, 1655–1668. [Google Scholar] [CrossRef]
- Ciabatti, I.; Femoni, C.; Gaboardi, M.; Iapalucci, M.C.; Longoni, G.; Pontiroli, D.; Riccò, M.; Zacchini, S. Structural rearrangements induced by acid-base reactions in metal carbonyl clusters: The case of [H3-nCo15Pd9C3(CO)38]n– (n = 0–3). Dalton Trans. 2014, 43, 4388–4399. [Google Scholar] [CrossRef]
- Berti, B.; Ciabatti, I.; Femoni, C.; Iapalucci, M.C.; Zacchini, S. Cluster Core Isomerism Induced by Crystal Packing Effects in the [HCo15Pd9C3(CO)38]2– Molecular Nanocluster. ACS Omega 2018, 3, 13239–13250. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heinemann: Oxford, UK, 1984. [Google Scholar]
- Melnik, M.; Mikus, P. Stereochemistry of heterobinuclear Pt-M complexes. Rev. Inorg. Chem. 2017, 37, 131–146. [Google Scholar] [CrossRef]
- Berti, B.; Bortoluzzi, M.; Cesari, C.; Femoni, C.; Iapalucci, M.C.; Mazzoni, R.; Vacca, F.; Zacchini, S. Polymerization Isomerism in [{MFe(CO)4}n]n– (M = Cu, Ag, Au; N 0 = 3, 4) Molecular Clusters Supported by Metallophilic Interactions. Inorg. Chem. 2019, 58, 2911–2915. [Google Scholar] [CrossRef] [PubMed]
- Cervellino, A.; Maspero, A.; Masciocchi, N.; Guagliardi, A. From ParacrystallineRu(CO)4 1D Polymer to Nanosized Ruthenium Metal: A Case of Study through Total Scattering Analysis. Cryst. Growth Des. 2012, 12, 3631–3637. [Google Scholar] [CrossRef]
- Klüfers, P. Preparation and Crystal Structure of CuCo(CO)4: A Carbonyl heterometal Complex Containing a Cu4Co4-Ring. Angew. Chem. Int. Ed. 1984, 23, 307–308. [Google Scholar] [CrossRef]
- Klüfers, P. [CuCo(CO)4]n—Crystal Structure of a Polymer with a One-Dimensional, Infinite Copper-Cobalt Bonding System. Angew. Chem. Int. Ed. 1985, 24, 70–71. [Google Scholar] [CrossRef]
- Klüfers, P. Die Kristallstruktur von AgCo(CO)4. Z. Krist. Cryst. Mater. 1984, 166, 143–151. [Google Scholar] [CrossRef]
- Banerjee, S.; Karunananda, M.K.; Bagherzadeh, S.; Jayarathne, U.; Parmelee, S.R.; Waldhart, G.W.; Mankad, N.P. Synthesis and Characterization of Heterobimetallic Complexes with Direct Cu-M Bonds (M = Cr, Mn, Co, Mo, Ru, W) Supported by N-Heterocyclic Carbene Ligands: A Toolkit for Catalytic Reaction Discovery. Inorg. Chem. 2014, 53, 11307–11315. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Chao, C.S.; Reibenspies, J.H.; Bischoff, C.J. Crystal structure and reactivity of bis[bis(1,2-dimethylphosphino)ethane]copper(2+) bis(tetracarbonylcobalt)cuprate(2−): Staggered and eclipsed conformations of [(CO)4CoCuCo(CO)4]– anions. Inorg. Chem. 1990, 29, 2153–2157. [Google Scholar] [CrossRef]
- Fuchs, R.; Klüfers, P. HeteronukleareKomplexverbindungenmitMetall-MetallBindungen, V. Umsetzungenmit [(NH3)2CuCo(CO)4]: Synthese und Struktur von [(PPh3)2CuCo(CO)4], [Cu{P(OMe)3}4][Cu{Co(CO)4}2] und (Ph3P)2N[Cu{Co(CO)4}2]. Z. Naturforsch. B 1991, 46, 507–518. [Google Scholar] [CrossRef]
- Usón, R.; Laguna, A.; Laguna, M.; Jones, P.G.; Sheldrick, G.M. Novel compounds with gold-transition-metal bonds; crystal and molecular structure of bis(triphenylphosphine)iminiumbis(tetracarbonylcobaltio)aurate(I). J. Chem. Soc. Dalton Trans. 1981, 2, 366–370. [Google Scholar] [CrossRef]
- Chini, P.; Martinengo, S.; Longoni, G. Synthesis of the trinuclear anions {Cu[Co(CO)4]2}– and {Ag[Co(CO)4]2}–. Gazz. Chim. Ital. 1975, 105, 203–204. [Google Scholar]
- Bowles, F.L.; Olmstead, M.M.; Beavers, C.M.; Balch, A.L. Reshaping molecules through cocrystallization. Comparison of the structures of Hg{Co(CO)4}2 and Hg{Co(CO)4}2·C60·toluene. Chem. Commu. 2013, 49, 5921–5923. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic Interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Berti, B.; Bortoluzzi, M.; Cesari, C.; Femoni, C.; Iapalucci, M.C.; Mazzoni, R.; Zacchini, S. A Comparative Experimental and Computational Study of Heterometallic Fe-M (M = Cu, Ag, Au) Carbonyl Clusters Containing N-Heterocyclic Carbene Ligands. Eur. J. Inorg. Chem. 2020, 2020, 2191–2202. [Google Scholar] [CrossRef]
- Berti, B.; Bortoluzzi, M.; Cesari, C.; Femoni, C.; Iapalucci, M.C.; Soleri, L.; Zacchini, S. Synthesis, Structural Characterization, and DFT Investigations of [MxM’5-xFe4(CO)16]3– (M, M’ = Cu, Ag, Au; M ≠ M’) 2-D Molecular Alloy Clusters. Inorg. Chem. 2020, 59, 15936–15952. [Google Scholar] [CrossRef]
- Blundell, T.L.; Powell, H.M. Stereochemistry of cobalt complexes with heteronuclear metal-metal bonds: The crystal and molecular structure of tetracarbonyl(triphenylphosphineaurio)cobalt, and of tetracarbonyl{[bis-(o-dimethylarsinophenyl)methylarsine]argentio}cobalt. J. Chem. Soc. A 1971, 1685–1690. [Google Scholar] [CrossRef]
- Femoni, C.; Iapalucci, M.C.; Longoni, G.; Tiozzo, C.; Wolowska, J.; Zacchini, S.; Zazzaroni, E. New Hybrid Semiconductor Materials Based on Viologen Salts of Bimetallic Fe-Pt and Fe-Au Carbonyl Clusters: First Structural Characterization of the Diradical π-Dimer of the Diethylviologen Monocation and EPR Evidence of its Triplet State. Chem. Eur. J. 2007, 13, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Mastorilli, P.; Nobile, C.F.; Braunstein, P.; Englert, U. Chelating versus bridging bonding modes of N-substituted bis(diphenylphosphanyl)amine ligands in Pt complexes and Co2Pt clusters. Dalton Trans. 2006, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Schnepf, A. Ge2Co6(CO)20: A Ge-Co Cluster Compound from Solubilized GeBr. Z. Anorg. Allg. Chem. 2007, 633, 938–940. [Google Scholar] [CrossRef]
- McCrea-Hendrick, M.L.; Caputo, C.A.; Roberts, C.J.; Fettinger, J.C.; Tuonnen, H.M.; Power, P.P. Reactions of Terphenyl-Substituted Digallene AriPr4GaGaAriPr4 (AriPr4 = C6H3-2,6-(C6H3-2,6-iPr2)2) with Transition Metal Carbonyls and Theoretical Investigation of the Mechanism of Addition. Organometallics 2016, 35, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Fachinetti, G.; Pucci, S.; Zanussi, P.F.; Methong, U. Redox-Additions/Eliminierungs-Reaktionen von Cluster-gebundenenLiganden: Oxidative Eliminierung von CO ausCo3(CO)9C-OH und reductive Addition von Acetylen an Co3(CO)9H. Angew. Chem. 1979, 91, 657–658. [Google Scholar] [CrossRef]
- Bradamante, P.; Pino, P.; Stefani, A.; Fachinetti, G.; Zanazzi, P.F. Presence of a metal-bonded hydrogen atom in unsaturated trinuclear cobalt clusters. Crystal structure of HCo3(CO)6(PPh3)3. J. Organomet. Chem. 1983, 251, C47–C50. [Google Scholar] [CrossRef]
- Klein, H.-F.; Mager, M.; Flörke, U.; Haupt, H.-J.; Breza, M.; Boca, R. Triangulo Cluster Molecules of Cobalt(0) and Nickel(0) Containing Trimethylphosphine and Carbonyl Ligands: Syntheses, Properties, and X-ray Structures. Organometallics 1992, 11, 2912–2916. [Google Scholar] [CrossRef]
- Klein, H.-F.; Mager, M.; Schmidt, A.; Hüber, M.; Haase, W.; Flörke, U.; Haupt, H.-J.; Boca, R. Synthesis, Structure, and Magnetic Properties of Low-ValentTriangulo Cobalt-Hydride Clusters [XCo3(μ-CO)3(PMe3)6]. Inorg. Chem. 1997, 36, 4303–4306. [Google Scholar] [CrossRef]
- Adams, H.-N.; Fachinetti, G.; Strahle, J. Distribution of Terminal and Bridging CO-Groups in an Anionic Carbonylmetal Cluster as a Result of Ion-Pair Formation. Crystal and Molecular Stricture of LiCo3(CO)10·i-Pr2O. Angew. Chem. Int. Ed. 1980, 19, 404–405. [Google Scholar] [CrossRef]
- Albano, V.G.; Chini, P.; Scatturin, V. Crystal and molecular structures of new cobalt carbonyl clusters. Chem. Commun. 1968, 163–164. [Google Scholar] [CrossRef]
- Edgell, W.F.; Lyford, J. The Preparation of Sodium Cobalt Tetracarbonyl. Inorg. Chem. 1970, 9, 1932–1933. [Google Scholar] [CrossRef]
- Kubas, G.J. Tetrakis(acetonitrile)copper(1+) hexafluorophosphate(1–). Inorg. Synth. 1990, 28, 68–70. [Google Scholar]
- Dash, K.C.; Schmidbaur, H. Organogold-Chemie, XII. Komplexe von Gold(I)- und Gold(III)- halogenidemmitThioäthern. Chem. Ber. 1973, 106, 1221–1225. [Google Scholar] [CrossRef]
- Keller, E. SCHAKAL99; University of Freiburg: Freiburg, Germany, 1999. [Google Scholar]
- Sheldrick, G.M. SADABS-2008/1—Bruker AXS Area Detector Scaling and Absorption Correction; Bruker AXS: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density−Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy−Adjusted ab initio Pseudopotentials for the First Row Transition Elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations-potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; DeFrees, D.J.; Pople, J.A.; Gordon, M.S. Self-Consistent Molecular Orbital Methods. 23. A polarization-type basis set for 2nd-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.J., III (Ed.) NIST Computational Chemistry Comparison and Benchmark Database; NIST Standard Reference Database Number 101; Release 21 August 2020; The National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2020. Available online: http://cccbdb.nist.gov/ (accessed on 1 February 2021).
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]













| 2 a | 3 b | 4 c | 5 d | 5 e | 5 f | 5 g | |
|---|---|---|---|---|---|---|---|
| M-Cot h | 2.393(3)–2.411(3) Av. 2.402(6) | 2.4392(17)–2.4450(17) Av. 2.442(5) | 2.363(5)–2.517(6) Av. 2.449(12) | 2.6537(3) | 2.6692(7) | 2.67936(18) | 2.669(4) and 2.679(4) |
| M-Cob h | - | - | - | 2.8576(3) | 2.8094(10) | 2.75393(19) and 2.8475(2) | 2.787(4)–2.805(4) Av. 2.798(8) |
| M-M | - | - | - | 2.8425(3) | 2.8775(7) | 2.91970(17) | 2.916(4) and 2.923(4) |
| Cot-M-Cot h | 180.0 | 180 | 180 | - | - | - | |
| Cot-M-M | - | - | - | 174.595(9) | 177.210(13) | 171.915(6) | 175.50(16) and 177.28(15) |
| M-Cob-M h | - | - | - | 60.725(7) | 61.951(12) | 62.806(4) | 63.08(11) and 62.75(11) |
| Cob-M-Cob h | - | - | - | 119.275(7) | 118.048(12) | 117.194(5) | 116.93(11) and 117.25(11) |
| Cob-M-M h | - | - | - | 61.273(7) | 59.51(2) | 57.031(5) and 60.163(5) | 58.20(10) and 58.47(11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesari, C.; Berti, B.; Calcagno, F.; Femoni, C.; Garavelli, M.; Iapalucci, M.C.; Rivalta, I.; Zacchini, S. Polymerization Isomerism in Co-M (M = Cu, Ag, Au) Carbonyl Clusters: Synthesis, Structures and Computational Investigation. Molecules 2021, 26, 1529. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26061529
Cesari C, Berti B, Calcagno F, Femoni C, Garavelli M, Iapalucci MC, Rivalta I, Zacchini S. Polymerization Isomerism in Co-M (M = Cu, Ag, Au) Carbonyl Clusters: Synthesis, Structures and Computational Investigation. Molecules. 2021; 26(6):1529. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26061529
Chicago/Turabian StyleCesari, Cristiana, Beatrice Berti, Francesco Calcagno, Cristina Femoni, Marco Garavelli, Maria Carmela Iapalucci, Ivan Rivalta, and Stefano Zacchini. 2021. "Polymerization Isomerism in Co-M (M = Cu, Ag, Au) Carbonyl Clusters: Synthesis, Structures and Computational Investigation" Molecules 26, no. 6: 1529. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26061529