Supramolecular Frameworks Based on Rhenium Clusters Using the Synthons Approach
Abstract
:1. Introduction
2. Experimental
2.1. Synthetic Methods
2.1.1. Preparation of the Hexarhenium(III) Clusters
2.1.2. Synthesis of Compounds 1 to 4
2.1.3. Synthesis of Compound 5
2.2. Crystal Structure Determinations
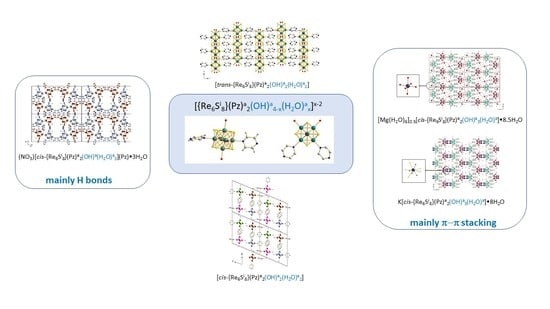
3. Results and Discussion
3.1. Crystal Structure Descriptions
3.1.1. Supramolecular Synthons Concept
3.1.2. A Common Cluster-Based Building Block
3.1.3. Crystal Structures of Compounds 1 to 5
Compound (1)
Compound (2)
Compound (3)
Compound (4)
Compound (5)
3.2. Supramolecular Synthons as Structural Keys for the Comparison of The Crystal Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naumov, N.G.; Virovets, A.V.; Fedorov, V.E. Octahedral rhenium(III) chalcocyanide cluster anions: Synthesis, structure, and solid state design. J. Struct. Chem. 2000, 41, 499–520. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Boubekeur, K.; Uriel, S.; Batail, P. Chemistry of Hexanuclear Rhenium Chalcohalide Clusters. Chem. Rev. 2001, 101, 2037–2066. [Google Scholar] [CrossRef]
- Selby, H.D.; Roland, B.K.; Zheng, Z. Ligand-Bridged Oligomeric and Supramolecular Arrays of the Hexanuclear Rhenium Selenide Clusters−Exploratory Synthesis, Structural Characterization, and Property Investigation. Acc. Chem. Res. 2003, 36, 933–944. [Google Scholar] [CrossRef]
- Perrin, A.; Perrin, C. The molybdenum and rhenium octahedral cluster chalcohalides in solid state chemistry: From condensed to discrete cluster units. C. R. Chim. 2012, 15, 815–836. [Google Scholar] [CrossRef]
- Cordier, S.; Molard, Y.; Brylev, K.A.; Mironov, Y.V.; Grasset, F.; Fabre, B.; Naumov, N.G. Advances in the Engineering of Near Infrared EmittingLiquid Crystals and Copolymers, Extended Porous Frameworks, Theranostic Tools and Molecular Junctions Using Tailored Re6 Cluster Building Blocks. J. Clust. Sci. 2015, 26, 53–81. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, V. Metal Clusters. As They Were Born in Siberia. J. Clust. Sci. 2015, 26, 3–15. [Google Scholar] [CrossRef]
- Shores, M.P.; Beauvais, L.G.; Long, J.R. [Cd2(H2O)4][Re6S8(CN)6].14H2O: A Cyano-Bridged Cluster-Cluster Framework Solid with Accessible Cubelike Cavities. Inorg. Chem. 1999, 38, 1648–1649. [Google Scholar] [CrossRef]
- Shores, M.P.; Beauvais, L.G.; Long, J.R. Cluster-Expanded Prussian Blue Analogues. J. Am. Chem. Soc. 1999, 121, 775–779. [Google Scholar] [CrossRef]
- Bennett, M.V.; Shores, M.P.; Beauvais, L.G.; Long, J.R. Expansion of the Porous Solid Na2Zn3[Fe(CN)6]2·9H2O: Enhanced Ion-Exchange Capacity in Na2Zn3[Re6Se8(CN)6]2·24H2O. J. Am. Chem. Soc. 2000, 122, 6664–6668. [Google Scholar] [CrossRef]
- Brylev, K.A.; Naumov, N.G.; Virovets, A.V.; Kim, S.-J.; Fedorov, V.E. Novel Three-Dimensional Coordination Polymers Based on [Mo6Se8(CN)6]7− Anions and Mn2+ Cations. J. Clust. Sci. 2009, 20, 165–176. [Google Scholar] [CrossRef]
- Bennett, M.V.; Beauvais, L.G.; Shores, M.P.; Long, J.R. Expanded Prussian Blue Analogues Incorporating [Re6Se8(CN)6]3−/4− Clusters: Adjusting Porosity via Charge Balance. J. Am. Chem. Soc. 2001, 123, 8022–8032. [Google Scholar] [CrossRef]
- Daigre, G.; Costuas, K.; Tarasenko, M.S.; Ledneva, A.Y.; Naumov, N.G.; Lemoine, P.; Guizouarn, T.; Molard, Y.; Amela-Cortes, M.; Audebrand, N.; et al. Stabilization of Ni2+ dimers in hexacyano Mo6 cluster-based Prussian blue derivatives: Experimental and theoretical investigations of magnetic properties. Dalton Trans. 2018, 47, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Brylev, K.A.; Mironov, Y.V.; Kozlova, S.G.; Fedorov, V.E.; Kim, S.-J.; Pietzsch, H.-J.; Stephan, H.; Ito, A.; Ishizaka, S.; Kitamura, N. The First Octahedral Cluster Complexes With Terminal Formate Ligands: Synthesis, Structure, and Properties of K4[Re6S8(HCOO)6] and Cs4[Re6S8(HCOO)6]. Inorg. Chem. 2009, 48, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Brylev, K.A.; Mironov, Y.V.; Fedorov, V.E.; Kim, S.-J.; Pietzsch, H.-J.; Stephan, H.; Ito, A.; Kitamura, N. A new hexanuclear rhenium cluster complex with six terminal acetate ligands: Synthesis, structure, and properties of K4[Re6S8(CH3COO)6]·8H2O. Inorg. Chim. Acta 2010, 363, 2686–2691. [Google Scholar] [CrossRef]
- Zheng, Z.; Long, J.R.; Holm, R.H. A Basis Set of Re6Se8 Cluster Building Blocks and Demonstration of Their Linking Capability: Directed Synthesis of an Re12Se16 Dicluster. J. Am. Chem. Soc. 1997, 119, 2163–2171. [Google Scholar] [CrossRef]
- Willer, M.W.; Long, J.R.; McLauchlan, C.C.; Holm, R.H. Ligand Substitution Reactions of [Re6S8Br6]4−: A Basis Set of Re6S8 Clusters for Building Multicluster Assemblies. Inorg. Chem. 1998, 37, 328–333. [Google Scholar] [CrossRef]
- Shestopalov, M.A.; Mironov, Y.V.; Brylev, K.A.; Kozlova, S.G.; Fedorov, V.E.; Spies, H.; Pietzsch, H.-J.; Stephan, H.; Geipel, G.; Bernhard, G. Cluster Core Controlled Reactions of Substitution of Terminal Bromide Ligands by Triphenylphosphine in Octahedral Rhenium Chalcobromide Complexes. J. Am. Chem. Soc. 2007, 129, 3714–3721. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Shestopalov, M.A.; Brylev, K.A.; Khlestkin, V.K.; Mironov, Y.V. A family of octahedral rhenium cluster complexes trans-[{Re6Q8}(PPh3)4X2] (Q = S or Se, X = Cl, Br or I): Preparation and halide-dependent luminescence properties. Polyhedron 2014, 81, 634–638. [Google Scholar] [CrossRef]
- Litvinova, Y.M.; Gayfulin, Y.M.; Kovalenko, K.A.; Samsonenko, D.G.; van Leusen, J.; Korolkov, I.V.; Fedin, V.P.; Mironov, Y.V. Multifunctional Metal–Organic Frameworks Based on Redox-Active Rhenium Octahedral Clusters. Inorg. Chem. 2018, 57, 2072–2084. [Google Scholar] [CrossRef]
- Litvinova, Y.M.; Gayfulin, Y.M.; van Leusen, J.; Samsonenko, D.G.; Lazarenko, V.A.; Zubavichus, Y.V.; Kögerler, P.; Mironov, Y.V. Metal–organic frameworks based on polynuclear lanthanide complexes and octahedral rhenium clusters. Inorg. Chem. Front. 2019, 6, 1518–1526. [Google Scholar] [CrossRef]
- Zheng, Z. Chemical transformations supported by the [Re6(μ3-Se)8]2+ cluster core. Daton Trans. 2012, 41, 5121–5131. [Google Scholar] [CrossRef]
- Grandubert, A.; Brylev, K.A.; Nguyen, T.T.; Naumov, N.G.; Kitamura, N.; Molard, Y.; Gautier, R.; Cordier, S. Synthesis and Crystal Structure of the Azide K4[Re6Sei8(N3)a6]·4H2O; Luminescence, Redox, and DFT Investigations of the [Re6Sei8(N3)a6]4− Cluster Unit. Z. Anorg. Allg. Chem. 2013, 639, 1756–1762. [Google Scholar]
- Mironov, Y.V.; Shestopalov, M.A.; Brylev, K.A.; Yarovoi, S.S.; Romanenko, G.V.; Fedorov, V.E.; Spies, H.; Pietzsch, H.-J.; Stephan, H.; Geipel, G.; et al. [Re6Q7O(3,5-Me2PzH)6]Br2·3,5-Me2PzH (Q = S, Se) − New Octahedral Rhenium Cluster Complexes with Organic Ligands: Original Synthetic Approach and Unexpected Ligand Exchange in the Cluster Core. Eur. J. Inorg. Chem. 2005, 657–661. [Google Scholar] [CrossRef]
- Mironov, Y.V.; Brylev, K.A.; Shestopalov, M.A.; Yarovoi, S.S.; Fedorov, V.E.; Spies, H.; Pietzsch, H.-J.; Stephan, H.; Geipel, G.; Bernhard, G.; et al. Octahedral rhenium cluster complexes with organic ligands: Synthesis, structure and properties of [Re6Q8(3,5-Me2PzH)6]Br2 · 2(3,5-Me2PzH) (Q = S, Se). Inorg. Chim. Acta 2006, 359, 1129–1134. [Google Scholar] [CrossRef]
- Mironov, Y.V.; Brylev, K.A.; Smolentsev, A.I.; Ermolaev, A.V.; Kitamura, N.; Fedorov, V.E. New mixed-ligand cyanohydroxo octahedral cluster complex trans-[Re6S8(CN)2(OH)4]4−, its luminescence properties and chemical reactivity. RSC Adv. 2014, 4, 60808–60815. [Google Scholar] [CrossRef]
- Shestopalov, M.A.; Zubareva, K.E.; Khripko, O.P.; Khripko, Y.I.; Solovieva, A.O.; Kuratieva, N.V.; Mironov, Y.V.; Kitamura, N.; Fedorov, V.E.; Brylev, K.A. The First Water-Soluble Hexarhenium Cluster Complexes with a Heterocyclic Ligand Environment: Synthesis, Luminescence, and Biological Properties. Inorg. Chem. 2014, 53, 9006–9013. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, D.I.; Ivanov, A.A.; Vorotnikov, Y.A.; Smolentsev, A.I.; Eltsov, I.V.; Efremova, O.A.; Kitamura, N.; Mironov, Y.V.; Shestopalov, M.A. Octahedral chalcogenide rhenium cluster complexes with imidazole. Polyhedron 2019, 165, 79–85. [Google Scholar] [CrossRef]
- Yoshimura, T.; Umakoshi, K.; Sasaki, Y.; Ishizaka, S.; Kim, H.-B.; Kitamura, N. Emission and Metal- and Ligand-Centered-Redox Characteristics of the Hexarhenium(III) Clusters trans- and cis-[Re6(μ3-S)8Cl4(L)2]2−, Where L Is a Pyridine Derivative or Pyrazine. Inorg. Chem. 2000, 39, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Dorson, F.; Molard, Y.; Cordier, S.; Fabre, B.; Efremova, O.; Rondeau, D.; Mironov, Y.; Cîrcu, V.; Naumov, N.; Perrin, C. Selective functionalisation of Re6 cluster anionic units: From hexa-hydroxo [Re6Q8(OH)6]4− (Q = S, Se) to neutral trans-[Re6Q8L4L′2] hybrid building blocks. Dalton Trans. 2009, 8, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Suo, C.; Tsuge, K.; Ishizaka, S.; Nozaki, K.; Saaki, Y.; Kitamura, N.; Shinohara, A. Excited-State Properties of Octahedral Hexarhenium(III) Complexes with Redox-active N-heteroaromatic Ligands. Inorg. Chem. 2010, 49, 531–540. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ishizaka, S.; Kashiwa, T.; Ito, A.; Sakuda, E.; Shinohara, A.; Kitamura, N. Direct Observation of a {Re6(μ3-S)8} Core-to-Ligand Charge-Transfer Excited State in an Octahedral Hexarhenium Complex. Inorg. Chem. 2011, 50, 9918–9920. [Google Scholar] [CrossRef]
- Ledneva, A.Y.; Naumov, N.G.; Virovets, A.V.; Cordier, S.; Molard, Y. Crystal structures of trans-[Re6S8(CN)2L4] complexes, L = pyridine or 4-methylpyridine. J. Struct. Chem. 2012, 53, 132–137. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Khlestkin, V.K.; Brylev, K.A.; Eltsov, I.V.; Smolentsev, A.I.; Mironov, Y.V.; Shestopalov, M.A. Synthesis, structure and luminescence properties of new chalcogenide octahedral rhenium cluster complexes with 4-aminopyridine [{Re6Q8}(4-NH2-py)6]2+. J. Coord. Chem. 2016, 69, 841–850. [Google Scholar] [CrossRef]
- El Osta, R.; Demont, A.; Audebrand, N.; Molard, Y.; Nguyen, T.T.; Gautier, R.; Brylev, K.A.; Mironov, Y.V.; Naumov, N.G.; Kitamura, N.; et al. Supramolecular Frameworks Built up from Red-Phosphorescent trans-Re6Cluster Building Blocks: One Pot Synthesis, Crystal Structures, and DFT Investigations. Z. Anorg. Alleg. Chem. 2015, 641, 1156–1163. [Google Scholar] [CrossRef]
- Kim, Y.; Fedorov, V.Z.; Jim, S.-J. Novel compounds based on [Re6Q8(L)6]4− (Q = S, Se, Te; L = CN, OH) and their applications. J. Mater. Chem. 2009, 19, 7178–7190. [Google Scholar] [CrossRef]
- Litvinova, Y.M.; Gayfulin, Y.M.; Samsonenko, D.G.; Bogomyakov, A.S.; Hyuk Shon, W.; Kim, S.-J.; Rhyee, J.-S.; Mironov, Y.V. Ladder coordination polymers built from [{Re4Q4(CN)12]4− cluster anions (Q = S, Se, Te) and [Gd(phen)(H2O)3Gd(phen)(H2O)2(μ-OH)2]4+ dimeric cationic fragments. Polyhedron 2016, 115, 174–179. [Google Scholar] [CrossRef]
- Mironov, Y.V.; Naumov, N.G.; Brylev, K.A.; Efremova, O.A.; Fedorov, V.E.; Hegetschweiler, K. Rhenium-chalcogenide-cyano clusters, Cu(2+) ions, and 1,2,3,4-tetraaminobutane as molecular building blocks for chiral coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 1297. [Google Scholar] [CrossRef] [PubMed]
- Brylev, K.A.; Mironov, Y.V.; Naumov, N.G.; Fedorov, V.E.; Ibers, J.A. New Compounds from Tellurocyanide Rhenium Cluster Anions and 3d-Transition Metal Cations Coordinated with Ethylenediamine. Inorg. Chem. 2004, 43, 4833–4838. [Google Scholar] [CrossRef] [PubMed]
- Brylev, K.A.; Pilet, G.; Naumov, N.G.; Perrin, A.; Fedorov, V.E. Structural Diversity of Low-Dimensional Compounds in [M(en)2]2+/[Re6Q8(CN)6]4− Systems (M = Mn, Ni, Cu). Eur. J. Inorg. Chem. 2005, 2005, 461–466. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.-M.; Nam, W.; Kim, S.-J. Crystal structure of the two-dimensional framework [Mn(salen)]4n[Re6Te8(CN)6]n [salen = N,N′-ethylenebis(salicylideneaminato)]. Chem. Comm. 2001, 16, 1470–1471. [Google Scholar] [CrossRef]
- Shestopalov, M.A.; Cordier, S.; Hernandez, O.; Molard, Y.; Perrin, C.; Perrin, A.; Fedorov, V.E.; Mironov, Y.V. Self-Assembly of Ambivalent Organic/Inorganic Building Blocks Containing Re6 Metal Atom Cluster: Formation of a Luminescent Honeycomb, Hollow, Tubular Metal-Organic Framework. Inorg. Chem. 2009, 48, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Shestopalov, M.A.; Ivanov, A.A.; Smolentsev, A.I.; Mironov, Y.V. Crystal structure of the octahedral cluster complex trans-[{Re6S8}(pyz)4I2]·2pyz. J. Struct. Chem. 2014, 55, 139–141. [Google Scholar] [CrossRef]
- Brylev, K.A.; Mironov, Y.V.; Yarovoi, S.S.; Naumov, N.G.; Fedorov, V.E.; Kim, S.-J.; Kitamura, N.; Kuwahara, Y.; Yamada, K.; Ishizaka, S.; et al. A Family of Octahedral Rhenium Cluster Complexes [Re6Q8(H2O)n(OH)6 - n]n - 4 (Q = S, Se; n = 0−6): Structural and pH−Dependent Spectroscopic Studies. Inorg. Chem. 2007, 46, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Yarovoi, S.S.; Mironov, Y.V.; Naumov, D.Y.; Gatilov, Y.V.; Kozlova, S.G.; Kim, S.-J.; Fedorov, V.E. Octahedral Hexahydroxo Rhenium Cluster Complexes [Re6Q8(OH)6]4–·(Q = S, Se): Synthesis, Structure, and Properties. Eur. J. Inorg. Chem. 2005, 19, 3945–3949. [Google Scholar] [CrossRef]
- APEX2 Program Suite V2014.11-0; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. St. Version 8.37a2013; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Sheldrick, G.M. Sadabs Version 2014/5 SADABS; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2002. [Google Scholar]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J. General methods for the construction of complex molecules. Pure Appl. Chem. 1967, 14, 19–37. [Google Scholar] [CrossRef]
- Pénicaud, A.; Boubekeur, K.; Batail, P.; Canadell, E. Hydrogen-bond tuning of macroscopic transport properties from the neutral molecular component site along the sries of metallic organic-inorganic solvates (BEDT-TTF)4Re6Se5Cl9.[guest], [guest = DMF, THF, dioxane]. J. Am. Chem. Soc. 1993, 115, 4101–4112. [Google Scholar] [CrossRef]
- Abu-Dari, K.; Raymond, K.N.; Freyberg, D.P. The bihydroxide (H3O2−) anion. A very short, symmetric hydrogen bond. J. Am. Chem. Soc. 1979, 101, 3688–3689. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen bridges in crystal engineering: Interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Bino, A.; Gibson, D. A new bridging ligand, the hydrogen oxide ion (H3O2−). J. Am. Chem. Soc. 1981, 103, 6741–6742. [Google Scholar] [CrossRef]
- Bino, A.; Gibson, D. The hydrogen oxide bridging ligand (H3O2−). 1. Dimerization and polymerization of hydrolyzed trinuclear metal cluster ions. J. Am. Chem. Soc. 1982, 104, 4383–4388. [Google Scholar] [CrossRef]
- Bino, A.; Gibson, D. The hydrogen oxide bridging ligand (H3O2−). 2. Effect of the hydrogen ion concentration. Inorg. Chem. 1984, 23, 109–115. [Google Scholar] [CrossRef]
- Adron, M.; Bino, A. Role of the H3O2 bridging ligand in coordination chemistry. 1. Structure of hydroxoaquametal ions. Inorg. Chem. 1985, 24, 1343–1347. [Google Scholar]
- D’Vries, R.F.; de la Pena-O’Shea, V.A.; Snejko, N.; Iglesias, M.; Gutierrez-Puebla, E.; Monge, M.A. H3O2 Bridging Ligand in a Metal–Organic Framework. Insight into the Aqua-Hydroxo ↔ Hydroxyl Equilibrium: A Combined Experimental and Theoretical Study. J. Am. Chem. Soc. 2013, 135, 5782–5792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledneva, A.Y.; Brylev, K.A.; Smolentsev, A.I.; Mironov, Y.V.; Molard, Y.; Cordier, S.; Kitamura, N.; Naumov, N.G. Controlled synthesis and luminescence properties of trans-[Re6S8(CN)4(OH)2−n(H2O)n]n−4 octahedral rhenium(III) cluster units (n = 0, 1 or 2). Polyhedron 2014, 67, 351–359. [Google Scholar] [CrossRef]
- Nagashima, S.; Furukawa, S.; Kamiguchi, S.; Kajio, R.; Nagashima, H.; Yamaguchi, A.; Shirai, M.; Kurokawa, H.; Chihara, T. Catalytic Activity of Molecular Rhenium Sulfide Clusters [Re6S8(OH)6−n(H2O)n](4−n)− (n = 0, 2, 4, 6) with Retention of the Octahedral Metal Frameworks: Dehydrogenation and Dehydration of 1,4-Butanediol. J. Clust. Sci. 2014, 25, 1203–1224. [Google Scholar] [CrossRef]
- Zheng, Z.; Selby, H.D.; Roland, B.K. The first ‘hexa-aqua-’ complex of the [Re6Se8]2+ cluster core, [Re6Se8(OH)2(H2O)4]·12H2O. Acta Cryst. 2001, E57, i77–i79. [Google Scholar]
- Mironov, Y.V.; Fedorov, V.E.; Bang, H.; Kim, S.-J. The First Coordination Polymers Based on Octahedral Hexahydroxo Rhenium Cluster Complexes [Re6Q8(OH)6]4– (Q = S, Se) and Alkaline Earth Metal Cations. Eur. J. Inorg. Chem. 2006, 553–557. [Google Scholar] [CrossRef]
- Uriel, S.; Boubekeur, K.; Gabriel, J.-C.; Batail, P.; Orduna, J. A concave, cubic diamond coordination polymer in the versatile chemistry of the aqua-alkaline-earth complex salts of molecular hexanuclear chalcohalide rhenium clusters, [Ca(H2O)n]Re6Q6Cl8.mH2O and [Mg(H2O)6]Re6S6Cl8.2H2O (Q = S, Se). Bull. Soc. Chim. Fr. 1996, 133, 783–794. [Google Scholar]










| Empirical Formula | (1) C8H8N4O6Re6S8 (trans) | (2) C8H8N4O4Re6S8 (cis) | (3) C12H12N7O10Re6S8 | (4) C16H15N8O30.10MgRe12S16 | (5) C8H8N4O12KRe6S8 |
|---|---|---|---|---|---|
| Formula weight/g.mol−1 | 1597.86 | 1597.86 | 1787.97 | 3572.71 | 1764.96 |
| Crystal description | prism | prism | Stick | plate | prism |
| Crystal color | orange | orange | yellow | orange | orange |
| Crystal size/mm3 | 0.035 × 0.030 × 0.025 | 0.1 × 0.06 × 0.05 | 0.09 × 0.02 × 0.015 | 0.1 × 0.08 × 0.02 | 0.14 × 0.08 × 0.03 |
| Crystal system | orthorhombic | triclinic | monoclinic | monoclinic | monoclinic |
| Space group | F2dd (No 43) | P(No 2) | C2/c (No 15) | P21/c (No 14) | C2/c (No 15) |
| a/Å | 10.5588(8) | 9.1971(4) | 31.1128(15) | 18.3212(5) | 33.8720 (8) |
| b/Å | 15.3154(12) | 18.7101(7) | 16.0496(9) | 12.6878(3) | 12.6850(3) |
| c/Å | 40.2137(31) | 19.9656(9) | 16.5582(7) | 30.1328(4) | 18.2242(5) |
| α/° | 90.00 | 69.606(2) | 90.00 | 90.00 | 90.00 |
| β/° | 90.00 | 84.114(2) | 115.788(2) | 96.7635(12) | 114.746(2) |
| γ/° | 90.00 | 88.969(2) | 90.00 | 90.00 | 90.00 |
| Volume/Å3 | 6503.0(9) | 3202.7(2) | 7444.9(6) | 7110.6(3) | 7111.3(3) |
| Z | 8 | 4 | 8 | 4 | 8 |
| T/K | 150 | 150 | 150 | 150 | 150 |
| ρ(calcd.)/g·cm–3 | 3.264 | 3.314 | 3.190 | 3.337 | 3.297 |
| μ/mm–1 | 22.778 | 23.125 | 19.929 | 20.882 | 20.978 |
| F(000) | 5552 | 2776 | 6328 | 6303 | 6216 |
| θ range/° | 3.344 to 39.867 | 2.893 to 32.532 | 2.48 to 21.47 | 2.889 to 27.484 | 2.45 to 27.48 |
| Collected reflections | 21,131 | 42,407 | 25,810 | 104,690 | 27,818 |
| Independent reflections | 8219 | 22,614 | 8508 | 16,121 | 8152 |
| Observed reflections [I > 2σ(I)] | 4425 | 8786 | 3331 | 11,385 | 6229 |
| No. restraints/No. refined parameters | 4/138 | 0/398 | 0/353 | 0/762 | 0/353 |
| Goodness-of-fit on F2 | 0.946 | 0.899 | 0.987 | 1.066 | 1.047 |
| R1, wR2 | 0.0491, 0.1009 | 0.0536, 0.1148 | 0.0642, 0.1251 | 0.0423, 0.0850 | 0.0393, 0.1073 |
| R1, wR2 (all data) | 0.1206, 0.1228 | 0.1567, 0.1494 | 0.2056, 0.1647 | 0.0741, 0.0988 | 0.0574, 0.1193 |
| Larg. diff. peak and hole/e·Å–3 | 2.645, −1.880 | 2.496, −2.462 | 2.227, −1.789 | 2.618, −2.061 | 3.265, −1.894 |
| Compound | Cluster Unit [{Re6Si8}(Pz)a2(OH)a4−x(H2O)ax]x−2 | H3O2− Bridge | H Bond | NO3− Bridge | π-π Stacking |
|---|---|---|---|---|---|
| (1) | trans-{Re6Si8}(Pz)a2(OH)a2(H2O)a2 | Strong H bond between cluster units → 2D framework | H bond between terminal nitrogen atoms of pirazine of cluster unit and equatorial oxygen atom from another cluster unit → 1D connectivity | ||
| (2) | cis-{Re6Si8}(Pz)a2(OH)a2(H2O)a2 | Strong H bond between cluster units along a axis → chains//a | H bond between terminal nitrogen atoms of pirazine of cluster unit and oxygen atom from another cluster unit → clusters tetramers, 2D framework | ||
| Strong H bond between cluster units perpendicular to a axis → 2D framework//(bc) | |||||
| (3) | [cis-{Re6Si8}(Pz)a2(OH)a(H2O)a3] | Strong H bonds between one free pyrazine group and two cluster units → 2D connectivity//(ab) of the chains, dimers | H bonds between one free nitrate group to two cluster units → chains//b | π-π stacking between pyrazine groups → cluster dimers | |
| H bonds between crystallization molecules themselves and also with O atoms from cluster units and nitrate groups | |||||
| (4) | [cis-Re6Si8(Pz)a2(OH)a3(H2O)a] | Strong H bond between O atoms of adjacent cluster units → 1D connectivity//c axis, cluster dimers | H bonds involving crystallization water molecules and water molecules connected to Mg2+ cation | π–π stacking between both aromatic rings of one cluster unit to connect with two different adjacent cluster units → chains//a | |
| π–π stacking between both aromatic rings of one cluster unit with only one another cluster unit belonging to another chains → cluster dimers, stacking of the chains along b axis | |||||
| (5) | [cis-Re6Si8(Pz)a2(OH)a3(H2O)a] | Strong H bond between O atoms of adjacent cluster units → 1D connectivity//a axis, cluster dimers | H bonds involving crystallization water molecules and water molecules connected to K+ cation | π–π stacking between both aromatic rings of one cluster unit to connect with two different adjacent cluster units → chains//c | |
| π–π stacking between both aromatic rings of one cluster unit with only one another cluster unit belonging to another chains → cluster dimers, stacking of the chains along b axis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audebrand, N.; Demont, A.; El Osta, R.; Mironov, Y.V.; Naumov, N.G.; Cordier, S. Supramolecular Frameworks Based on Rhenium Clusters Using the Synthons Approach. Molecules 2021, 26, 2662. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092662
Audebrand N, Demont A, El Osta R, Mironov YV, Naumov NG, Cordier S. Supramolecular Frameworks Based on Rhenium Clusters Using the Synthons Approach. Molecules. 2021; 26(9):2662. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092662
Chicago/Turabian StyleAudebrand, Nathalie, Antoine Demont, Racha El Osta, Yuri V. Mironov, Nikolay G. Naumov, and Stéphane Cordier. 2021. "Supramolecular Frameworks Based on Rhenium Clusters Using the Synthons Approach" Molecules 26, no. 9: 2662. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092662