Outlining In Vitro and In Silico Cholinesterase Inhibitory Activity of Twenty-Four Natural Products of Various Chemical Classes: Smilagenin, Kokusaginine, and Methyl Rosmarinate as Emboldening Inhibitors
Abstract
:1. Introduction
2. Results
2.1. Evaluation of ChE Inhibitory Activities of the Tested Compounds
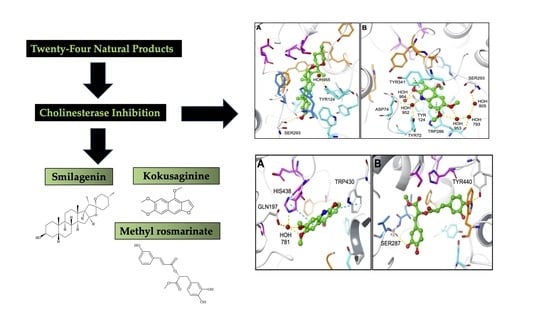
2.2. Molecular Docking Data for the Inhibitory Compounds
3. Discussion
4. Materials and Methods
4.1. Isolation of the Tested Compounds
4.2. Microtiter Assays for Cholinesterase Inhibition
4.3. Data Processing for Enzyme Inhibition Assays
4.4. Molecular Docking Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Oboudiyat, C.; Glazer, H.; Seifan, A.; Greer, C.; Isaacson, R.S. Alzheimer’s disease. Semin. Neurol. 2013, 33, 313–329. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar]
- Serý, O.; Povová, J.; Míšek, I.; Pešák, L.; Janout, V. Molecular mechanisms of neuropathological changes in Alzheimer’s disease: A review. Folia Neuropathol. 2013, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimers Dis. 2017, 60, 401–425. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar]
- Massoulié, J. The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals 2002, 11, 130–143. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef]
- Marco, L.; do Carmo Carreiras, M. Galanthamine, a natural product for the treatment of Alzheimer’s disease. Recent Pat. Cns Drug Discov. 2006, 1, 105–111. [Google Scholar] [CrossRef]
- Gulcan, H.O.; Orhan, I.E.; Sener, B. Chemical and molecular aspects on interactions of galanthamine and its derivatives with cholinesterases. Curr. Pharm. Biotechnol. 2015, 16, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Senol Deniz, F.S.; Eren, G.; Sener, B. Molecular approach to promising cholinesterase inhibitory effect of several Amaryllidaceae alkaloids: Further re-investigation. S. Afr. J. Bot. 2021, 136, 175–181. [Google Scholar] [CrossRef]
- Pinho, B.R.; Ferreres, F.; Valentão, P.; Andrade, P.B. Nature as a source of metabolites with cholinesterase-inhibitory activity: An approach to Alzheimer’s disease treatment. J. Pharm. Pharm. 2013, 65, 1681–1700. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Su, T.; Li, X. Natural products as sources of new lead compounds for the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2013, 13, 1864–1878. [Google Scholar] [CrossRef]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangez, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Satheeshkumar, N.; Venkatesh, P.; Venkatesh, M. Lead finding for acetyl cholinesterase inhibitors from natural origin: Structure activity relationship and scope. Mini Rev. Med. Chem. 2011, 11, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Orhan, G.; Gurkas, E. An overview on natural cholinesterase inhibitors -a multi-targeted drug class- and their mass production. Mini Rev. Med. Chem. 2011, 11, 836–842. [Google Scholar] [CrossRef]
- Orhan, I.E. Implications of some selected flavonoids towards Alzheimer’s disease with the emphasis on cholinesterase inhibition and their bioproduction by metabolic engineering. Curr. Pharm. Biotechnol. 2014, 15, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Gulcan, H.O.; Orhan, I.E. A recent look into natural products that have potential to inhibit cholinesterases and monoamine oxidase B: Update on 2010-2019. Comb. Chem. High Throughput Screen. 2020, 23, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Habartová, K.; Cahlíková, L.; Řezáčová, M.; Havelek, R. The biological activity of alkaloids from the Amaryllidaceae: From cholinesterases ınhibition to anticancer activity. Nat. Prod. Commun. 2016, 11, 1587–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Kabir, M.T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular ınsight into the therapeutic promise of flavonoids against Alzheimer’s disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghayur, M.N.; Kazim, S.F.; Rasheed, H.; Khalid, A.; Jumani, M.I.; Choudhary, M.I.; Gilani, A.H. Identification of antiplatelet and acetylcholinesterase inhibitory constituents in betel nut. Zhong Xi Yi Jie He Xue Bao 2011, 9, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Muthusamy, K.; Niranjan, M.; Trikha, S.; Kumar, S. Sarsasapogenin: A steroidal saponin from Asparagus racemosus as multi target directed ligand in Alzheimer’s disease. Steroids 2020, 153, 108529. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Howson, P.A.; Xia, Z.; Zhou, S.; Wu, E.; Xia, Z.; Hu, Y. Smilagenin attenuates beta amyloid (25–35)-induced degeneration of neuronal cells via stimulating the gene expression of brain-derived neurotrophic factor. Neuroscience 2012, 210, 275–285. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Z.; Sun, Q.; Orsi, A.; Rees, D. A new approach to the pharmacological regulation of memory: Sarsasapogenin improves memory by elevating the low muscarinic acetylcholine receptor density in brains of memory-deficit rat models. Brain Res. 2005, 1060, 26–39. [Google Scholar] [CrossRef]
- Kiełczewska, U.; Jorda, R.; Gonzalez, G.; Morzycki, J.W.; Ajani, H.; Svrčková, K.; Štěpánková, Š.; Wojtkielewicz, A. The synthesis and cholinesterase inhibitory activities of solasodine analogues with seven-membered F ring. J. Steroid Biochem. Mol. Biol. 2021, 205, 105776. [Google Scholar] [CrossRef]
- Santoro, V.; Parisi, V.; D’Ambola, M.; Sinisgalli, C.; Monné, M.; Milella, L.; Russo, R.; Severino, L.; Braca, A.; Tommasi, N.D. Chemical profiling of Astragalus membranaceus roots (Fish.) Bunge herbal preparation and evaluation of its bioactivity. Nat. Prod. Commun. 2020, 15, 1–11. [Google Scholar]
- Li, W.Z.; Wu, W.Y.; Huang, D.K.; Yin, Y.Y.; Kan, H.W.; Wang, X.; Yao, Y.Y.; Li, W.P. Protective effects of astragalosides on dexamethasone and Aβ25–35 induced learning and memory impairments due to decrease amyloid precursor protein expression in 12-month male rats. Food Chem. Toxicol. 2012, 50, 1883–1890. [Google Scholar]
- Chang, C.P.; Liu, Y.F.; Lin, H.J.; Hsu, C.C.; Cheng, B.C.; Liu, W.P.; Lin, M.T.; Hsu, S.F.; Chang, L.S.; Lin, K.C. Beneficial effect of astragaloside on Alzheimer’s disease condition using cultured primary cortical cells under β-amyloid exposure. Mol. Neurobiol. 2016, 53, 7329–7340. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Muñoz, E.; Salazar, J.R.; Yamaguchi, L.; Werner, E.; Alarcon, J.; Kubo, I. Inhibition of cholinesterase activity by extracts, fractions and compounds from Calceolaria talcana and C. integrifolia (Calceolariaceae: Scrophulariaceae). Food Chem. Toxicol. 2013, 62, 919–926. [Google Scholar] [CrossRef]
- Vo, T.N.; Nguyen, P.L.; Tuong, L.T.; Vo, P.N.; Nguyen, K.P.P.; Nguyen, N.S. Constituents of the leaves of Pseuderanthemum carruthersii (Seem.) Guill. var. atropurpureum (Bull.) Fosb. Phytochem. Lett. 2012, 5, 673–676. [Google Scholar] [CrossRef]
- Kahraman, C.; Tatli, I.I.; Orhan, I.E.; Akdemir, Z.S. Cholinesterase ınhibitory and antioxidant properties of Verbascum mucronatum Lam. and its secondary metabolites. Z. Naturforsch. Sect. C 2010, 65, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Alipieva, K.; Orhan, I.; Abrashev, R.; Denev, P.; Angelova, M. Antioxidant and cholinesterase inhibitory activities of Verbascum xanthophoeniceum and its phenylethanoid glycosides. Food Chem. 2011, 128, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Lopes, E.M.; Maier, J.A.; da Silva, M.R.; Regasini, L.O.; Simote, S.Y.; Lopes, N.P.; Pirani, J.R.; da Silva Bolzani, V.; Young, M.C.M. Alkaloids from stems of Esenbeckia leiocarpa Engl. (Rutaceae) as potential treatment for Alzheimer disease. Molecules 2010, 15, 9205–9213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sichaem, J.; Rojpitikul, T.; Sawasdee, P.; Lugsanangarm, K.; Tip-Pyang, S. Furoquinoline alkaloids from the leaves of Evodia lepta as potential cholinesterase ınhibitors and their molecular docking. Nat. Prod. Commun. 2015, 10, 1359–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, I.; Aslan, S.; Kartal, M.; Sener, B.; Başer, K.H.C. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008, 108, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids-interaction testing in model solutions. Ann. Agric. Env. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef]
- Senol, F.S.; Ślusarczyk, S.; Matkowski, A.; Pérez-Garrido, A.; Girón-Rodríguez, F.; Cerón-Carrasco, J.P.; den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H.; Domaradzki, K.; et al. Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia atriplicifolia Benth. and Salvia glutinosa L. Phytochemistry 2017, 133, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kocakaya, S.O.; Ertas, A.; Yener, I.; Ercan, B.; Oral, E.V.; Akdeniz, M.; Kaplaner, E.; Topcu, G.; Kolak, U. Selective in-vitro enzymes’ inhibitory activities of fingerprints compounds of Salvia species and molecular docking simulations. Iran J. Pharm. Res. 2020, 19, 187–198. [Google Scholar] [PubMed]
- Noyanalpan, N.; Sener, B.; Toker, G.; Tosun, F.; Türköz, S.; Nadir Sarışeker, N. Studies on utilizing the sapogenols of indigenous or cultivated plants of Anatolia for the synthesis of steroid medicinals. III. The sapogenols of Digitalis cariensis Boiss. Hacettepe Univ. Fac. Pharm. 1985, 5, 23–26. [Google Scholar]
- Sener, B.; Mutlugil, A.; Noyanalpan, N.; Lewis, J.R. Alkaloids from Haplophyllum myrtifolium Boiss. J. Fac. Pharm. Gazi 1990, 7, 17–24. [Google Scholar]
- Woo, E.R.; Piao, M.S. Antioxidative constituents from Lycopus lucidus. Arch. Pharm. Res. 2004, 27, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Zor, M.; Saracoglu, I.; Isimer, A.; Rüegger, H. Four novel cycloartane glycosides from Astragalus oleifolius. J. Nat. Prod. 1996, 59, 1019–1023. [Google Scholar] [CrossRef]
- Calis, I.; Yuruker, A.; Tasdemir, D.; Wright, A.D.; Sticher, O.; Luo, Y.D.; Pezzuto, J.M. Cycloartane triterpene glycosides from the roots of Astragalus melanophrurius. Planta Med. 1997, 63, 183–186. [Google Scholar] [CrossRef]
- Calis, I.; Yusufoglu, H.; Zerbe, O.; Sticher, O. Cephalotoside A, a tridesmosidic cycloartane type glycoside from Astragalus cephalotes var. brevicalyx. Phytochemistry 1999, 49, 732–736. [Google Scholar]
- Ozipek, M.; Donmez, A.A.; Calis, I.; Brun, R.; Rüedi, P.; Tasdemir, D. Leishmanicidal cycloartane-type triterpene glycosides from Astragalus oleifolius. Phytochemistry 2005, 66, 1168–1173. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Zou, K.; Luo, H.; Wang, J.; Huang, N. Induced-fit docking and virtual screening for 8- hydroxy- 3-methoxy5H-pyrido[2,1-c]pyrazin-5-one derivatives as inducible nitric oxide synthase inhibitors. J. Chem. Pharm. Res. 2014, 6, 1187–1194. [Google Scholar]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Brus, B.; Kosak, V.; Turk, S.; Pislar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nano- molar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef]




| Compounds Tested | Chemical Class | ChE Inhibition (Inhibition % ± S.D.a at 100 µg/mL) | |
|---|---|---|---|
| AChE | BChE | ||
| Diosgenin | Steroidal saponins | 4.31 ± 0.37 | 8.12 ± 2.64 |
| Hecogenin | 25.36 ± 1.11 | 17.30 ± 1.84 | |
| Rockogenin | 5.29 ± 0.52 | 15.23 ± 2.56 | |
| Smilagenin | 62.41 ± 2.44 (IC50 = 43.29 ± 1.38 µg/mL) | 15.04 ± 3.01 | |
| Tigogenin | 17.09 ± 0.69 | 22.23 ± 3.09 | |
| Astrasieversianin II | Cycloartane triterpenes | 8.01 ± 0.48 | 13.56 ± 1.79 |
| Astrasieversianin X | 8.60 ± 0.91 | 10.06 ± 2.34 | |
| Astragaloside I | -b | 8.38 ± 1.28 | |
| Astragaloside IV | 4.80 ± 0.05 | 6.62 ± 2.72 | |
| Astragaloside VI | 6.23 ± 2.82 | 7.72 ± 0.53 | |
| Cyclocanthoside E | 2.18 ± 1.81 | 5.35 ± 1.82 | |
| Cyclocanthoside G | 6.85 ± 0.72 | 8.23 ± 0.24 | |
| Macrophyllosaponin A | 9.69 ± 0.85 | 12.17 ± 1.83 | |
| Macrophyllosaponin B | 13.21 ± 0.92 | 12.16 ± 1.16 | |
| Macrophyllosaponin C | 12.42 ± 1.40 | 16.49 ± 1.57 | |
| Macrophyllosaponin D | 4.31 ± 1.32 | 10.10 ± 0.78 | |
| Kokusaginine | Alkaloid | 62.35 ± 3.20 (IC50 = 70.24 ± 2.87 µg/mL) | 67.43 ± 3.10 (IC50 = 61.40 ± 3.67 µg/mL) |
| Lamiide | Iridoid | - | 8.77 ± 0.92 |
| Ipolamide | Iridoid | - | 12.44 ± 2.28 |
| Forsythoside B | Phenylpropanoid | - | 14.80 ± 2.31 |
| Verbascoside | Phenylpropanoid | 8.12 ± 1.49 | 22.24 ± 2.54 |
| Alyssonoside | Phenylpropanoid | - | 12.99 ± 2.07 |
| Methyl rosmarinate | Phenolic acid ester | 16.79 ± 3.84 | 82.74 ± 0.62 (IC50 = 41.46 ± 2.83 μg/mL) |
| Luteolin-7-O-glucuronide | Flavonoid heteroside | - | - |
| Galanthamine HBr (Reference) | 94.19 ± 0.31 (IC50 = 1.33 ± 0.11 µg/mL) | 60.30 ± 1.36 (IC50 = 52.31 ± 3.04 µg/mL) | |
| Compound | Glide Score (kcal/mol) | Interacting Residues and Interaction Types |
|---|---|---|
| Smilagenin | −12.10 | TYR124 (HOH955 mediated H-bond) SER293 (H-bond) |
| Kokusaginine | −10.44 | TYR72 (HOH952 mediated H-bond) ASP74 (HOH952 mediated H-bond) TYR124 (HOH954 mediated H-bond) TRP286 (π-π stack and HOH953, HOH793 mediated H-bond) SER293 (HOH953, HOH793, HOH805 mediated H-bond) TYR341 (π-π stack) |
| Methyl rosmarinate | −10.15 | TRP86 (π-π stack) TRP286 (π-π stack) |
| Hecogenin | −8.35 | TYR72 (HOH952 mediated H-bond) ASP74 (HOH952 mediated H-bond) TYR124 (HOH955 mediated H-bond) |
| Tigogenin | −7.73 | TRP286 (HOH953, HOH793 mediated H-bond) |
| Galanthamine | −11.86 | ASP74 (salt bridge) TRP86 (π-cation) GLU202 (H-bond) TYR337 (π-cation) PHE338 (π-cation and π-π stack) |
| Compound | Glide Score (kcal/mol) | Interacting Residues and Interaction Types |
|---|---|---|
| Smilagenin | −7.78 | TRP430 (H-bond) |
| Kokusaginine | −7.49 | GLU197 (HOH781 mediated H-bond) TRP430 (π-π stack) HIS438 (π-π stack and HOH781 mediated H-bond) |
| Methyl rosmarinate | −9.39 | SER287 (H-bond) TYR440 (H-bond) |
| Hecogenin | −8.08 | GLU197 (H-bond) |
| Tigogenin | −7.22 | GLU197 (HOH781 mediated H-bond) HIS438 (H-bond) |
| Galanthamine | −9.51 | TYR332 (π-cation) HIS438 (H-bond) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senol Deniz, F.S.; Eren, G.; Orhan, I.E.; Sener, B.; Ozgen, U.; Aldaba, R.; Calis, I. Outlining In Vitro and In Silico Cholinesterase Inhibitory Activity of Twenty-Four Natural Products of Various Chemical Classes: Smilagenin, Kokusaginine, and Methyl Rosmarinate as Emboldening Inhibitors. Molecules 2021, 26, 2024. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26072024
Senol Deniz FS, Eren G, Orhan IE, Sener B, Ozgen U, Aldaba R, Calis I. Outlining In Vitro and In Silico Cholinesterase Inhibitory Activity of Twenty-Four Natural Products of Various Chemical Classes: Smilagenin, Kokusaginine, and Methyl Rosmarinate as Emboldening Inhibitors. Molecules. 2021; 26(7):2024. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26072024
Chicago/Turabian StyleSenol Deniz, F. Sezer, Gokcen Eren, Ilkay Erdogan Orhan, Bilge Sener, Ufuk Ozgen, Randa Aldaba, and Ihsan Calis. 2021. "Outlining In Vitro and In Silico Cholinesterase Inhibitory Activity of Twenty-Four Natural Products of Various Chemical Classes: Smilagenin, Kokusaginine, and Methyl Rosmarinate as Emboldening Inhibitors" Molecules 26, no. 7: 2024. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26072024