Antioxidant, Anti-Inflammatory, and Inhibition of Acetylcholinesterase Potentials of Cassia timoriensis DC. Flowers
Abstract
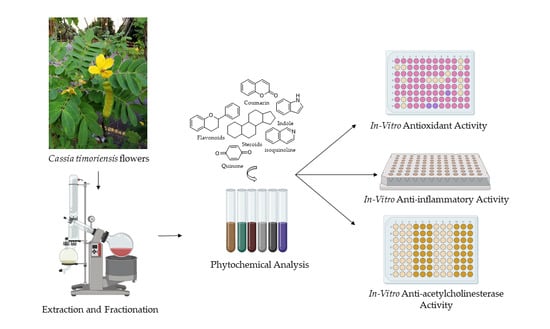
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening of Cassia timoriensis Flowers
2.2. Antioxidant Capacity of Cassia timoriensis Flower Extracts
2.3. Anti-Inflammatory Activity of Cassia timoriensis Flower Extracts
2.4. In Vitro Anti-Acetylcholinesterase Activity of Cassia timoriensis Flower Extracts
3. Materials and Methods
3.1. Materials (Chemicals)
3.2. Plant Collection and Identification
3.3. Plant Extraction and Fractionation
3.4. Phytochemical Screening
3.5. Antioxidant Capacity
3.5.1. Total Flavonoid Content (TFC)
3.5.2. Total Phenolic Content (TPC)
3.5.3. Radical Scavenging Capacity
3.6. Anti-Inflammatory Activity
3.7. Inhibition of Acetylcholinesterase Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive Substances of Plant Origin 30. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 967, pp. 967–1008. [Google Scholar]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Hunyadi, A. The mechanism (s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.A.; Costa, T.W.R.; Fonseca, A.C.D.; Amaral, R.F.D.; Desterro, S.M.D.; Santos-Filho, O.A.; Miranda, A.L.P.D.; Neto, D.C.F.; Lima, F.R.; Hamerski, L. Geissoschizoline, a promising alkaloid for Alzheimer’s disease: Inhibition of human cholinesterases, anti-inflammatory effects and molecular docking. Bioorg. Chem. 2020, 104, 104215. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Dandapat, J.; Dash, U.C.; Kanhar, S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018, 215, 42–73. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. J. Alzheimers Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Codina, C.; Bastida, J. The Genus Galanthus: A Source of Bioactive Compounds. In Phytochemicals-A Global Perspective of Their Role in Nutrition and Health; IntechOpen: London, UK, 2012. [Google Scholar]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.A.; Gad, H.; Radwan, R.; Ayoub, N.; El-Shazly, M. Chemical constituents and biological activities of Cassia genus: Review. Arch. Pharm. Sci. Ain Shams Univ. 2019, 3, 195–227. [Google Scholar]
- Hu, J.M.; Lavin, M.; Wojciechowski, M.F.; Sanderson, M.J. Phylogenetic systematics of the tribe Millettieae (Leguminosae) based on chloroplast trnK/matK sequences and its implications for evolutionary patterns in Papilionoideae. Am. J. Bot. 2000, 87, 418–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raes, N.; Saw, L.; Van Welzen, P.C.; Yahara, T. Legume diversity as indicator for botanical diversity on Sundaland, south east Asia. S. Afr. J. Bot. 2013, 89, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Lim, T. Senna timoriensis. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; pp. 886–888. [Google Scholar]
- Monkheang, P.; Sudmoon, R.; Tanee, T.; Noikotr, K.; Bletter, N.; Chaveerach, A. Species diversity, usages, molecular markers and barcode of medicinal Senna species (Fabaceae, Caesalpinioideae) in Thailand. J. Med. Plant Res. 2011, 5, 6173–6181. [Google Scholar] [CrossRef]
- Palasuwan, A.; Soogarun, S.; Lertlum, T.; Pradniwat, P.; Wiwanitkit, V. Inhibition of Heinz body induction in an invitro model and total antioxidant activity of medicinal Thai plants. APJCP 2005, 6, 458. [Google Scholar] [PubMed]
- Gritsanapan, W.; Tantisewie, B.; Jirawongse, V. Chemical constituents of Cassia timorensis and Cassia grandis. Sci. Asia 1984, 10, 189–190. [Google Scholar] [CrossRef]
- Azman, N.A.N.; Alhawarri, M.B.; Rawa, M.S.A.; Dianita, R.; Gazzali, A.M.; Nogawa, T.; Wahab, H.A. Potential anti-acetylcholinesterase activity of Cassia timorensis DC. Molecules 2020, 25, 4545. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Saeedi, M.; Nabavi, S.F.; Silva, A.S. Terpenes and terpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–496. [Google Scholar]
- Egbuna, C.; Ifemeje, J.C.; Udedi, S.C.; Kumar, S. Phytochemistry: Volume 1: Fundamentals, Modern Techniques, and Applications; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2018. [Google Scholar]
- Chaudhary, S.; Kumar, A. Phytochemical analysis and assessment of in-vitro anthelmintic activity of Cassia auriculata Linn leaves. Am. J. Phytomed. Clin. Therap. 2014, 2, 161–167. [Google Scholar]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Boukraa, D.; Belabid, L.; Benabdelli, K.; Bennabi, F. The effect of the salicylic acid on the variability of phenolic compounds, during the germination and the seedling of chickpea (Cicer arietinum L.), after inoculation by mushrooms. Eur. J. Biotechn. Biosci. 2014, 1, 27–35. [Google Scholar]
- Mansour, R.B.; Ksouri, W.M.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. Assessment of antioxidant activity and neuroprotective capacity on PC12 cell line of Frankenia thymifolia and related phenolic LC-MS/MS identification. Evid. Based Complement. Altern. Med. 2016, 2016, 2843463. [Google Scholar]
- Kolar, F.R.; Gogi, C.L.; Khudavand, M.M.; Choudhari, M.S.; Patil, S.B. Phytochemical and antioxidant properties of some Cassia species. Nat. Prod. Res. 2018, 32, 1324–1328. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.; Jiang, K.; Tao, S.; Li, Y.; Chen, W.; Kou, S.; Gu, C.; Li, Z.; Guo, L. A review of flavonoids from cassia species and their biological activity. Curr. Pharm. Biotechnol. 2016, 17, 1134–1146. [Google Scholar] [CrossRef]
- Juan-Badaturuge, M.; Habtemariam, S.; Thomas, M.J. Antioxidant compounds from a south Asian beverage and medicinal plant, Cassia auriculata. Food Chem. 2011, 125, 221–225. [Google Scholar] [CrossRef]
- Mahesh, V.; Sharma, R.; Singh, R.; Upadhya, S. Anthraquinones and kaempferol from Cassia species section fistula. J. Nat. Prod. 1984, 47, 733. [Google Scholar] [CrossRef]
- Panichayupakaranant, P.; Kaewsuwan, S. Bioassay-guided isolation of the antioxidant constituent from Cassia alata L. leaves. Songklanakarin J. Sci. Technol. 2004, 26, 103–107. [Google Scholar]
- Wahab, A.; Begum, S. Luteolin and kaempferol from Cassia alata, antimicrobial and antioxidant activity of its methanolic extracts. FUUAST J. Biol. 2014, 4, 1–5. [Google Scholar]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Indian J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- Lee, N.-H.; Lee, S.-M.; Song, D.-H.; Yang, J.-Y.; Lee, H.-S. Antimicrobial effect of emodin isolated from Cassia tora Linn. seeds against food-borne bacteria. Appl. Biol. Chem. 2013, 56, 187–189. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.M.; El-Souda, S.S.; El-Hallouty, S.M.; Kobayashi, N. Antiviral and cytotoxic activities of anthraquinones isolated from Cassia roxburghii Linn. leaves. Herba Pol. 2013, 59, 33–44. [Google Scholar] [CrossRef]
- Promgool, T.; Pancharoen, O.; Deachathai, S. Antibacterial and antioxidative compounds from Cassia alata Linn. Songklanakarin J. Sci. Technol. 2014, 36, 459–463. [Google Scholar]
- Kalidhar, S.B. Alatinone, an anthraquinone from Cassia alata. Phytochem. 1993, 32, 1616–1617. [Google Scholar]
- Sekar, M.; Prasad, K.R.; Sidduraju, P.; Janardhanan, K. New anthraquinones from Cassia obtusa. Fitoterapia 1999, 70, 330–332. [Google Scholar] [CrossRef]
- Kamagaté, M.; Koffi, C.; Kouamé, N.; Akoubet, A.; Alain, N.; Yao, R.; Die, H. Ethnobotany, phytochemistry, pharmacology and toxicology profiles of Cassia siamea Lam. J. Phytopharmacol. 2014, 3, 57–76. [Google Scholar]
- Wu, Q.; Wang, Z.; Fu, M.; Tang, L.; He, Y.; Fang, J.; Gong, Q. Chemical constituents from the leaves of Cassia angustifolia. Zhong Yao Cai 2007, 30, 1250–1252. [Google Scholar] [PubMed]
- Banjarnahor, S.D.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Kaurinovic, B.; Vastag, D. Flavonoids and phenolic acids as potential natural antioxidants. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Pourreza, N. Phenolic compounds as potential antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidèle, N.; Joseph, B.; Emmanuel, T.; Théophile, D. Hypolipidemic, antioxidant and anti-atherosclerogenic effect of aqueous extract leaves of Cassia occidentalis Linn (Caesalpiniaceae) in diet-induced hypercholesterolemic rats. BMC Complement. Altern. Med. 2017, 17, 76. [Google Scholar] [CrossRef] [Green Version]
- Ishak, I.F.A.R.; Lajis, H.M.; Ambia, K.M.; Noah, R.M. Effects of Cassia alata treatment towards cardiovascular oxidative stress in hyperglycemic rats. Int. J. Pharm. Sci. Rev. Res. 2015, 34, 254–258. [Google Scholar]
- Gupta, S.; Sharma, S.B.; Singh, U.R.; Bansal, S.K. Salutary effect of Cassia auriculata L. leaves on hyperglycemia-induced atherosclerotic environment in streptozotocin rats. Cardiovasc. Toxicol. 2011, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.S.; Kim, H.G.; Choi, J.G.; Ryu, J.H.; Hur, J.; Kim, Y.J.; Oh, M.S. Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson’s disease models. Food Chem. Toxicol. 2010, 48, 2037–2044. [Google Scholar] [CrossRef]
- Yi, J.H.; Park, H.J.; Lee, S.; Jung, J.W.; Kim, B.C.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. Cassia obtusifolia seed ameliorates amyloid β-induced synaptic dysfunction through anti-inflammatory and Akt/GSK-3β pathways. J. Ethnopharmacol. 2016, 178, 50–57. [Google Scholar] [CrossRef]
- Rejiya, C.; Cibin, T.; Abraham, A. Leaves of Cassia tora as a novel cancer therapeutic–An in vitro study. Toxicol. In Vitro 2009, 23, 1034–1038. [Google Scholar] [CrossRef]
- Padmalochana, K. Anticancer (liver cancer cell lines) and antioxidant activity of Cassia auriculata flower extract from acetone and methanol solvents. JDDT 2018, 8, 274–278. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Baskar, A.A.; Ignacimuthu, S.; Muthukumar, C.; Al-Harbi, N. Anticancer activity of Rhein isolated from Cassia fistula L. flower. Asian Pac. J. Trop. Dis. 2012, 2, S517–S523. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med. 2016, 16, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Jo, K. In-vitro valuation of anti-inflammatory effect of Panax Ginseng by inhibition of albumin denaturation experiment. APEC Youth Sci. J. 2017, 9, 1–5. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Bogdan, M.; Pirnau, A.; Floare, C.; Bugeac, C. Binding interaction of indomethacin with human serum albumin. J. Pharm. Biomed. 2008, 47, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L.; Hill, L.A.; Round, P.W. Roles of Plasma Binding Proteins in Modulation of Hormone Action and Metabolism. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Nicholson, J.; Wolmarans, M.; Park, G. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell Ther. 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.L.L.; Arráez-Román, D.; Segura-Carretero, A. Functional ingredients based on nutritional phenolics. A case study against inflammation: Lippia genus. Nutrients 2019, 11, 1646. [Google Scholar] [CrossRef] [Green Version]
- Brüll, F.; Mensink, R. Plant sterols: Functional lipids in immune function and inflammation? J. Clin. Lipidol. 2009, 4, 355–365. [Google Scholar] [CrossRef]
- Ntandou, G.N.; Banzouzi, J.; Mbatchi, B.; Elion-Itou, R.; Etou-Ossibi, A.; Ramos, S.; Benoit-Vical, F.; Abena, A.; Ouamba, J. Analgesic and anti-inflammatory effects of Cassia siamea Lam. stem bark extracts. J. Ethnopharmacol. 2010, 127, 108–111. [Google Scholar] [CrossRef]
- Basha, S.I.; Somashekara, S.; Govindadas, D.; Naidu, D.; Devasankaraiah, G.; Mohato, R.; Yadav, K. Anti-inflammatory activity of Cassia occidentalis seeds in albino rats. J. Nat. Pharm. 2011, 2, 88–91. [Google Scholar] [CrossRef]
- Gobianand, K.; Vivekanandan, P.; Pradeep, K.; Mohan, C.; Karthikeyan, S. Anti-inflammatory and antipyretic activities of Indian medicinal plant Cassia fistula Linn. (Golden Shower) in Wistar albino rats. Int. J. Pharmacol. 2010, 6, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, S.S.; Chaudhari, S.R.; Chavan, M.J. Analgesic, anti-inflammatory and anti-arthritic activity of Cassia uniflora Mill. Asian Pac. J. Trop. Biomed. 2012, 2, S181–S186. [Google Scholar] [CrossRef]
- Antonisamy, P.; Dhanasekaran, M.; Kim, H.-R.; Jo, S.-G.; Agastian, P.; Kwon, K.-B. Anti-inflammatory and analgesic activity of ononitol monohydrate isolated from Cassia tora L. in animal models. Saudi J. Biol. Sci. 2017, 24, 1933–1938. [Google Scholar] [CrossRef]
- Moriyama, H.; Iizuka, T.; Nagai, M.; Miyataka, H.; Satoh, T. Antiinflammatory activity of heat-treated Cassia alata leaf extract and its flavonoid glycoside. Yakugaku Zasshi 2003, 123, 607–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, A.; Rajalingam, D.; Maity, T.K. Anti-inflammatory effect of O-methylated flavonol 2-(3,4-dihydroxy-phenyl)-3,5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. J. Ethnopharmacol. 2013, 147, 525–529. [Google Scholar] [CrossRef]
- Antonisamy, P.; Agastian, P.; Kang, C.-W.; Kim, N.S.; Kim, J.-H. Anti-inflammatory activity of Rhein isolated from the flowers of Cassia fistula L. and possible underlying mechanisms. Saudi J. Biol. Sci. 2019, 26, 96–104. [Google Scholar] [CrossRef]
- Antwi, A.O.; Obiri, D.D.; Osafo, N.; Essel, L.B.; Forkuo, A.D.; Atobiga, C. Stigmasterol alleviates cutaneous allergic responses in rodents. BioMed Res. Int. 2018, 2018, 3984068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, N.-R.; Kim, H.-M.; Jeong, H.-J. The β-sitosterol attenuates atopic dermatitis-like skin lesions through down-regulation of TSLP. Exp. Biol. Med. 2014, 239, 454–464. [Google Scholar] [CrossRef]
- Donadu, M.G.; Le, N.T.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, M.; Marchetti, M.; Sanna, G.; Madeddu, S. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of Hornstedtia bella Škorničk. Antibiotics 2020, 9, 334. [Google Scholar] [CrossRef]
- Netopilova, M.; Houdkova, M.; Urbanova, K.; Rondevaldova, J.; Van Damme, P.; Kokoska, L. In vitro antimicrobial combinatory effect of Cinnamomum cassia essential oil with 8-hydroxyquinoline against Staphylococcus aureus in liquid and vapour phase. J. Appl. Microbiol. 2020, 129, 906–915. [Google Scholar] [CrossRef]
- Santos, T.C.D.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.D.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Nat. C 2007, 62, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids-interaction testing in model solutions. AAEM 2015, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.F.; Almeida, M.P.; Leite, M.F.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Amaral, J.G.; David, J.M. Seasonal variation in the chemical composition of two chemotypes of Lippia alba. Food Chem. 2019, 273, 186–193. [Google Scholar] [CrossRef]
- Derkach, T.M.; Starikova, O.O. Variation of chemical composition of medicinal herbs of different producers. J. Chem. Technol. 2019, 27, 79–91. [Google Scholar] [CrossRef]
- Shrestha, S.; Seong, S.H.; Paudel, P.; Jung, H.A.; Choi, J.S. Structure related inhibition of enzyme systems in cholinesterases and BACE1 in vitro by naturally occurring naphthopyrone and its glycosides isolated from Cassia obtusifolia. Molecules 2018, 23, 69. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Ali, M.Y.; Jung, H.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases. J. Ethnopharmacol. 2016, 191, 152–160. [Google Scholar] [CrossRef]
- Aftab, Z.; Khan, H.; Khan, A.; Ullah, H.; Khan, S. Three new cholinesterase inhibitory cassioates from Cassia fistula. Pharm. Chem. J. 2020, 53, 1069–1075. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Dey, P.; Chatterjee, P.; Chandra, S.; Bhattacharya, S. Comparative in vitro evaluation of anti-inflammatory effects of aerial parts and roots from Mikania scandens. J. Adv. Pharm. Educ. Res. 2011, 1, 271–277. [Google Scholar]
- Banerjee, S.; Chanda, A.; Adhikari, A.; Das, A.; Biswas, S. Evaluation of phytochemical screening and anti inflammatory activity of leaves and stem of Mikania scandens (L.) wild. Ann. Med. Health Sci. Res. 2014, 4, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Sarveswaran, R.; Jayasuriya, W.; Suresh, T. In vitro assays to investigate the anti-inflammatory activity of herbal extracts a review. World J. Pharm. Res. 2017, 6, 131–141. [Google Scholar]
- Rawa, M.S.A.; Hassan, Z.; Murugaiyah, V.; Nogawa, T.; Wahab, H.A. Anti-cholinesterase potential of diverse botanical families from Malaysia: Evaluation of crude extracts and fractions from liquid-liquid extraction and acid-base fractionation. J. Ethnopharmacol. 2019, 245, 112160. [Google Scholar] [CrossRef] [PubMed]
- Balkis, A.; Tran, K.; Lee, Y.Z.; Balkis, K.N.; Ng, K. Screening flavonoids for inhibition of acetylcholinesterase identified baicalein as the most potent inhibitor. J. Agric. Sci. 2015, 7, 26. [Google Scholar] [CrossRef] [Green Version]


| No. | Class | Test | HE | EE | ME | AE |
|---|---|---|---|---|---|---|
| 1 | Alkaloids | Mayer’s test | - | - | - | - |
| Wagner’s test | - | - | - | - | ||
| Dragendorff’s test | - | - | - | - | ||
| 2 | Flavonoids | Alkaline reagent test | + | + | + | - |
| Zn/HCL reduction test | + | + | + | - | ||
| 3 | Tannins | Ferric chloride test | + | + | + | + |
| 4 | Saponins | Frothing test | - | - | - | + |
| 5 | Cardiac glycosides | Keller–Killiani test | - | - | - | - |
| 6 | Anthraquinones glycoside | Borntrager’s test | - | + | - | - |
| 7 | Steroids | Liebermann–Burchard test | + | + | + | - |
| Salkowski test | + | + | + | + | ||
| 8 | Terpenoids | Modified Salkowski test | + | - | + | - |
| 9 | Coumarins | - | + | + | + | - |
| 10 | Quinones | - | - | - | - | - |
| 11 | Proteins | Millon’s test | - | + | + | + |
| 12 | Carbohydrates | Benedict’s test (reducing sugar) | - | + | + | + |
| Sample | TPC mg GAE/g DW | TFC mg QE/g DW | Antioxidant Activity (DPPH Assay) | |
|---|---|---|---|---|
| % Inhibition * | IC50 (µg/mL) | |||
| n-Hexane extract | 136.36 ± 9.58 | 300.58 ± 10.78 | 45.18 ± 0.51 | 54.08 ± 0.78 |
| Ethyl acetate extract | 527.43 ± 5.83 | 851.83 ± 10.08 | 97.80 ± 0.29 | 20.12 ± 0.12 |
| Methanol extract | 321.75 ± 11.33 | 493.92 ± 9.27 | 71.74 ± 0.39 | 34.48 ± 0.07 |
| Aqueous extract | 31.05 ± 7.94 | 61.83 ± 9.10 | 12.18 ± 2.58 | - |
| Ascorbic acid | - | - | 98.73 ± 0.25 | 20.22 ± 0.03 |
| Sample | Concentration (µg/mL) | % Inhibition ** |
|---|---|---|
| n-Hexane extract | 100 | 43.13 ± 2.63 |
| 200 | 85.25 ± 2.50 | |
| Ethyl acetate extract | 100 | 92.38 ± 0.74 |
| 200 | 92.50 ± 1.38 | |
| Methanol extract | 100 | 89.45 ± 1.25 |
| 200 | 92.22 ± 1.09 | |
| Aqueous extract | 100 | 36.76 ± 1.50 |
| 200 | 87.16 ± 2.02 | |
| Indomethacin | 100 | 90.04 ± 0.87 |
| 200 | 91.15 ± 0.32 |
| Sample | % Inhibition * | IC50 (µg/mL) |
|---|---|---|
| Galantamine | 98.64 ± 0.01 | 1.33 ± 0.03 |
| Aqueous extract | 38.32 ± 0.09 | - |
| Methanol extract | 96.55 ± 0.02 | 6.40 ± 0.27 |
| Ethyl acetate extract | 96.87 ± 0.05 | 6.91 ± 0.38 |
| n-Hexane extract | 92.35 ± 0.014 | 12.08 ± 0.95 |
| No. | Class | Test | Method | Positive Result | Ref. |
|---|---|---|---|---|---|
| 1 | Alkaloids | Mayer’s test | A few milligrams of each extract were dissolved individually in dilute HCL and filtered. Then, the filtrates were separately treated with Mayer’s, Wagner’s, and Dragendorff’s Reagents to test for the presence of alkaloids. | Turbidity or creamy precipitate | [19] |
| Wagner’s test | Yellow–brown precipitate | [19] | |||
| Dragendorff’s test | Turbidity or orange–red precipitate | [19] | |||
| 2 | Flavonoids | Alkaline test | About 2 mL of 20% NaOH solution was added to 1 mL of alcoholic solution of each plant extract individually. | Observation of intense yellow color | [19] |
| Zn/HCl test | A pinch of zinc dust added to 2 mL of the alcoholic solution of sample. Then, a few drops of concentrated HCL were added slowly. | Observation of pink to red color | [20] | ||
| 3 | Tannins | Ferric chloride test | About 10 mg of the extracts was boiled in 10 mL of water in a test tube and then filtered. Then, a few drops of 1% ferric chloride wew added to the filtrate. | Hydrolysable tannins give bluish-black color, while condensed give brownish-green color | [19] |
| 4 | Saponin | Frothing test | A few milligrams of each extract were mixed separately with 5 mL of distilled water and mixed vigorously. | Persistent foam | [19] |
| 5 | Cardiac glycoside | Keller–Killiani test | About 3 mg of each extract was dissolved in 3 mL of concentrated acetic acid. Then, one drop of 5% FeCl3 solution was added, followed by few drops of concentrated sulphuric acid. | A reddish-brown ring forms at the interface | [18,19] |
| 6 | Anthraquinone glycoside | Borntrager’s test | A few milligrams of each extract were treated with dilute HCL and boiled for 5 min, cooled, and shaken with an equal volume of chloroform, benzene, or any other organic layer; then, the organic layer was separated and treated with ammonia. | Pink to red color in aqueous alkaline layer | [19] |
| 7 | Steroids | Salkowski’s test | A few milligrams of sample were treated with chloroform and filtered. The filtrates were then treated with a few drops of concentrated sulfuric acid. | Greenish-yellow color indicates the presence of steroids | [18,19] |
| Liebermann–Burchard test | About 2 mg of each extract was dissolved in acetic anhydride, heated, and cooled before adding 1 mL of concentrated sulphuric acid along the test tube’s sides. | Green color indicates the presence of steroids nucleus | [18,19] | ||
| 8 | Triterpenoids | Modified Salkowoski’s test | About 1 mL of each of the four extracts was added to 1 mL of chloroform and filtered to clarify the solution, followed by dropwise addition of a few drops of concentrated sulphuric acid at the wall side of test tube. | Observation of reddish-brown color | |
| 9 | Coumarins | - | To 2 mL of each extract, a few drops of 10% alcoholic NaOH were added. | Observation of yellow color | [19] |
| 10 | Quinone | - | To 1 mL of each extract, a few drops of NaOH were added. | Observation of red or blue green color | [19] |
| 11 | Protein | Million’s test | A few drops of Million’s reagent were added to 2 mL of each sample and mixed. | Red color or precipitate indicated the presence of protein | [19] |
| 12 | Carbohydrate | Benedict’s test | A few drops of Benedict’s reagent were added to an aqueous solution of each plant extract and mixed. | Observation of orange–red color | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhawarri, M.B.; Dianita, R.; Razak, K.N.A.; Mohamad, S.; Nogawa, T.; Wahab, H.A. Antioxidant, Anti-Inflammatory, and Inhibition of Acetylcholinesterase Potentials of Cassia timoriensis DC. Flowers. Molecules 2021, 26, 2594. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092594
Alhawarri MB, Dianita R, Razak KNA, Mohamad S, Nogawa T, Wahab HA. Antioxidant, Anti-Inflammatory, and Inhibition of Acetylcholinesterase Potentials of Cassia timoriensis DC. Flowers. Molecules. 2021; 26(9):2594. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092594
Chicago/Turabian StyleAlhawarri, Maram B., Roza Dianita, Khairul Niza Abd Razak, Suriani Mohamad, Toshihiko Nogawa, and Habibah A. Wahab. 2021. "Antioxidant, Anti-Inflammatory, and Inhibition of Acetylcholinesterase Potentials of Cassia timoriensis DC. Flowers" Molecules 26, no. 9: 2594. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092594