Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats
Abstract
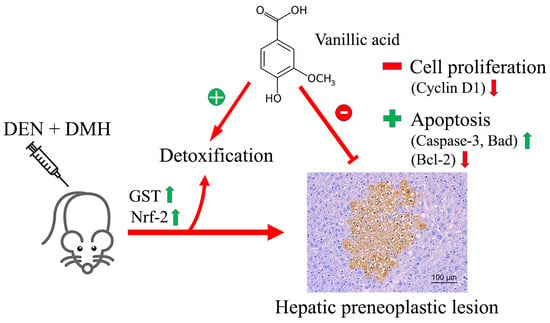
:1. Introduction
2. Results
2.1. Effect of VA on General Observations, Relative Organ Weights and Serum Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) in Diethylnitrosamine-(DEN) and 1,2-Dimethylhydrazine-(DMH) Initiated Rats
2.2. Effect of VA on Preneoplastic Lesion Formation in Livers and Colons of DEN- and DMH-Initiated Rats
2.3. Effect of VA on Cell Proliferation and Apoptosis
2.4. Effect of VA on the mRNA Levels of Genes Involved in the Early Stages of Hepatocarcinogenesis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals and Treatment Protocol
4.3. Determination of Serum AST and ALT Levels
4.4. Determination of Hepatic and Colonic Preneoplastic Lesions in Rats
4.5. Determination of PCNA and Apoptotic Hepatocytes by Double-Staining Immunohistochemistry
4.6. Gene Expression Analysis by Real-Time Polymerase Chain Reaction
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Patterson, A.D.; Gonzalez, F.J.; Perdew, G.H.; Peters, J.M. Molecular regulation of carcinogenesis: Friend and foe. Toxicol. Sci. 2018, 165, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Rejhova, A.; Opattova, A.; Cumova, A.; Sliva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, G.; Singh, C.K.; Ndiaye, M.A.; Fedorowicz, S.; Molot, A.; Ahmad, N. Prostate cancer chemoprevention by natural agents: Clinical evidence and potential implications. Cancer Lett. 2018, 422, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Weidner, S. Content of phenolic acids in rye caryopses determined using DAD-HPLC method. Czech J. Food Sci. 2001, 19, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Punvittayagul, C.; Sringarm, K.; Chaiyasut, C.; Wongpoomchai, R. Mutagenicity and antimutagenicity of hydrophilic and lipophilic extracts of Thai northern purple rice. Asian Pac. J. Cancer Prev. 2014, 15, 9517–9522. [Google Scholar]
- Sang, S.; Lapsley, K.; Jeong, W.S.; Lachance, P.A.; Ho, C.T.; Rosen, R.T. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Sevgi, K.; Tepe, B.; Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 2015, 77, 12–21. [Google Scholar] [CrossRef]
- Vinothiya, K.; Ashokkumar, N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017, 87, 640–652. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, S.J.; Kim, D.S.; Jeon, Y.D.; Park, S.J.; Lee, H.S.; Um, J.Y.; Hong, S.H. Vanillic acid inhibits inflammatory mediators by suppressing NF-kappaB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Stanely Mainzen Prince, P.; Rajakumar, S.; Dhanasekar, K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharmacol. 2011, 668, 233–240. [Google Scholar] [CrossRef]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin A-induced liver injury. Biol. Pharm. Bull. 2009, 32, 1215–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilnumkhum, A.; Punvittayagul, C.; Chariyakornkul, A.; Wongpoomchai, R. Effects of hydrophilic compounds in purple rice husk on AFB1-induced mutagenesis. Mol. Cell Toxicol. 2017, 13, 171–178. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Chewonarin, T.; Jarukamjorn, K.; Wongpoomchai, R. Augmentation of diethylnitrosamine-induced early stages of rat hepatocarcinogenesis by 1,2-dimethylhydrazine. Drug Chem. Toxicol. 2019, 42, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Sayavongsa, P.; Cooper, M.L.; Jackson, E.M.; Harris, L.; Ziegler, T.R.; Hibbert, J.M. Vanillic acid excretion can be used to assess compliance with dietary supplements. e-Spen. Eur. e-J. Clin. Nutr. Metab. 2007, 2, e134–e137. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.S.; Wanibuchi, H.; Morimura, K.; Gonzalez, F.J.; Fukushima, S. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Res. 2007, 67, 11141–11146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, D.W.; Giardina, C.; Tanaka, T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 2009, 30, 183–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar]
- Keum, Y.S. Regulation of Nrf2-mediated phase II detoxification and anti-oxidant genes. Biomol. Ther. 2012, 20, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Marí, M.; Cederbaum, A.I. Induction of catalase, alpha, and microsomal glutathione S-transferase in CYP2E1 overexpressing HepG2 cells and protection against short-term oxidative stress. Hepatology 2001, 33, 652–661. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 1–10. [Google Scholar]
- Alao, J.P. The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol. Cancer 2007, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Danial, N.N. BCL-2 family proteins: Critical checkpoints of apoptotic cell death. Clin. Cancer Res. 2007, 13, 7254–7263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Pietta, P.G.; Simonetti, P.; Gardana, C.; Brusamolino, A.; Morazzoni, P.; Bombardelli, E. Catechin metabolites after intake of green tea infusions. Biofactor 1998, 8, 111–118. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Sankam, P.; Wongpoomchai, R. Inhibitory effect of Thai purple rice husk extract on chemically induced carcinogenesis in rats. Molecules 2021, 26, 360. [Google Scholar] [CrossRef]
- Thumvijit, T.; Taya, S.; Punvittayagul, C.; Peerapornpisal, Y.; Wongpoomchai, R. Cancer chemopreventive effect of Spirogyra neglecta (Hassall) Kutzing on diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian Pac. J. Cancer Prev. 2014, 15, 1611–1616. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.L.; Zeng, T.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. Protective effects of garlic oil on hepatocarcinoma induced by N-nitrosodiethylamine in rats. Int. J. Biol. Sci. 2012, 8, 363–374. [Google Scholar] [CrossRef]
- Khan, M.; Halagowder, D.; Devaraj, S. Methylated chrysin induces coordinated attenuation of the canonical Wnt and NF-kB signaling pathway and upregulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chem. Biol. Interact. 2011, 193, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yeligar, S.M.; Machida, K.; Vijay, K.K. Ethanol-induced HO-1 and NQO1 are differentially regulated by HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression. J. Biol. Chem. 2010, 285, 35359–35373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Man, S.; Li, J.; Zhang, Y.; Meng, X.; Gao, W. Inhibition of diethylnitrosamine-induced liver cancer in rats by Rhizoma paridis saponin. Environ. Toxicol. Pharmacol. 2016, 46, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Badr, R.; Hashemi, M.; Javadi, G.; Movafagh, A.; Mahdian, R. Gene expression profiles of BAD and Bcl-xL in the CA1 region of the hippocampus following global ischemic/reperfusion and FK-506 administration. Iran Red. Crescent Med. J. 2015, 17, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Anarkooli, I.J.; Sankian, M.; Ahmadpour, S.; Varasteh, A.R.; Haghir, H. Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats. Exp. Diabetes Res. 2008, 2008, 1–6. [Google Scholar] [CrossRef]
- Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Limtrakul Dejkriengkraikul, P.; Wongpoomchai, R. Protective effects of defatted sticky rice bran extracts on the early stages of hepatocarcinogenesis in rats. Molecules 2019, 24, 2142. [Google Scholar] [CrossRef] [Green Version]






| Treatment | Week of Administration | Final Body Weight (g) | Relative Organ Weight (%) | AST (U/L) | ALT (U/L) | |||
|---|---|---|---|---|---|---|---|---|
| Carcinogen/NSS | VA (mg kg−1 BW) | Liver | Spleen | Kidney | ||||
| DEN + DMH | - | - | 398.8 ± 22.8 * | 3.35 ± 0.14 | 0.25 ± 0.03 | 0.57 ± 0.03 | 109.5 ± 25.7 * | 64.8 ± 19.1 * |
| DEN + DMH | 0.75 | 10 | 383.8 ± 19.8 | 3.26 ± 0.29 | 0.24 ± 0.02 | 0.55 ± 0.03 | 116.4 ± 29.0 | 61.6 ± 8.9 |
| DEN + DMH | 75 | 10 | 399.4 ± 35.8 | 3.19 ± 0.15 | 0.21 ± 0.02 | 0.56 ± 0.02 | 110.4 ± 29.4 | 57.6 ± 8.4 |
| DEN + DMH | 0.75 | 7 | 382.5 ± 13.4 | 3.38 ± 0.18 | 0.24 ± 0.02 | 0.57 ± 0.04 | 92.9 ± 12.1 | 54.3 ± 7.43 |
| DEN + DMH | 75 | 7 | 395.0 ± 21.9 | 3.35 ± 0.17 | 0.25 ± 0.04 | 0.55 ± 0.02 | 109.4 ± 21.8 | 62.7 ± 17.8 |
| NSS | - | - | 445.0 ± 37.4 | 3.40 ± 0.11 | 0.20 ± 0.02 | 0.55 ± 0.02 | 75.5 ± 15.6 | 39.2 ± 8.3 |
| NSS | 75 | 10 | 428.0 ± 31.7 | 3.40 ± 0.31 | 0.19 ± 0.02 | 0.56 ± 0.03 | 84.6 ± 20.9 | 43.0 ± 8.4 |
| Treatment | Week of Administration | Preneoplastic Lesion | ||||
|---|---|---|---|---|---|---|
| Carcinogen/NSS | VA (mg kg−1 BW) | Liver | Colon | |||
| No. of GST-P+ Foci/cm2 | Area (mm2/cm2) | Aberrant Crypt/Rat | Aberrant Crypt/Focus | |||
| DEN + DMH | - | - | 21.65 ± 8.07 * | 1.52 ± 0.72 * | 159.33 ± 31.51 * | 4.69 ± 0.75 * |
| DEN + DMH | 0.75 | 10 | 21.28 ± 6.73 | 1.58 ± 0.77 | 163.50 ± 29.40 | 4.03 ± 0.80 |
| DEN + DMH | 75 | 10 | 10.15 ± 4.60 ** | 0.59 ± 0.29 ** | 138.88 ± 14.93 | 3.87 ± 0.46 |
| DEN + DMH | 0.75 | 7 | 22.21 ± 7.47 | 1.62 ± 0.65 | 133.00 ± 25.69 | 4.31 ± 0.49 |
| DEN + DMH | 75 | 7 | 19.43 ± 4.50 | 1.36 ± 0.41 | 151.88 ± 14.80 | 4.12 ± 0.44 |
| NSS | - | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| NSS | 75 | 10 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Gene | 5′-3′ Primer Sequence | References |
|---|---|---|
| Bax | Forward: 5′-GTT GCC CTC TTC TAC TTT GC-3′ Reverse: 5′-ATG GTC ACT GTC TGC CAT G-3′ | [30] |
| Caspase-3 | Forward: 5′-CTG GAC TGC GGT ATT GAG AC-3′ Reverse: 5′-CCG GGT GCG GTA GAG TAA GC-3′ | [30] |
| Cyclin D1 | Forward: 5′-GTC GAG AAG AGA AAG CTC TG-3′ Reverse: 5′-TTA AAA GCC TCC TGT GTG AA-3′ | [31] |
| Nrf-2 | Forward: 5′-GCC AGC TGA ACT CCT TAG AC-3′ Reverse: 5′-GAT TCG TGC ACA GCA GCA-3′ | [32] |
| GSTA-5 | Forward: 5′-ACC CCT TTC CCT CTG CTG AA-3′ Reverse: 5′-AAA CAT CAG AGC CTG GAT TAC AAG-3′ | NM_001010921.1 |
| CYP2E1 | Forward: 5′-CCT TTC CCT CTT CCC ATC C-3′ Reverse: 5′-AAC CTC CGC ACA TCC TTC C-3′ | [33] |
| Bad | Forward: 5′-GGA GCA TCG TTC AGC AGC AG-3′ Reverse: 5′-CCA TCC CTT CAT CTT CCT CAG TC-3′ | [34] |
| Bcl-2 | Forward: 5′-CTG GTG GAC AAC ATC GCT CTG-3′ Reverse: 5′-GGT CTG CTG ACC TCA CTT GTG-3′ | [35] |
| β-Actin | Forward: 5′-ACA GGA TGC AGA AGG AGA TTA C-3′ Reverse: 5′-AGA GTG AGG CCA GGA TAG A-3′ | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules 2021, 26, 2718. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092718
Punvittayagul C, Chariyakornkul A, Jarukamjorn K, Wongpoomchai R. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules. 2021; 26(9):2718. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092718
Chicago/Turabian StylePunvittayagul, Charatda, Arpamas Chariyakornkul, Kanokwan Jarukamjorn, and Rawiwan Wongpoomchai. 2021. "Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats" Molecules 26, no. 9: 2718. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092718