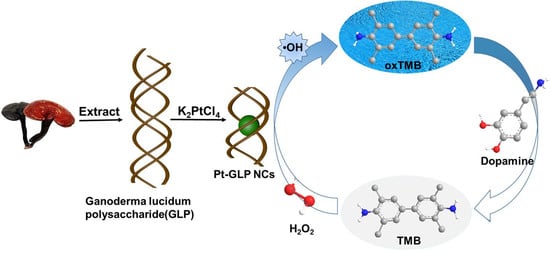
Peroxidase-Like Platinum Clusters Synthesized by Ganoderma lucidum Polysaccharide for Sensitively Colorimetric Detection of Dopamine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Characterizations
2.2. Peroxidase-Like Activity
2.3. Steady-State Kinetic
2.4. Catalytic Mechanism
2.5. Detection of Dopamine
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Extraction of Polysaccharide
3.3. Determination of Molecular Weight
3.4. Determination of Reducing Power
3.5. Purity Determination
3.6. Synthesis and Characterizations of Ptn-GLP NCs
3.7. Measurements for the Peroxidase-Like Catalytic Activity
3.8. Kinetic Analysis
3.9. Mechanism Detection
3.10. Dopamine Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Singh, R. Recent optical sensing technologies for the detection of various biomolecules: Review. Opt. Laser. Technol. 2021, 134, 106620. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, Y.; Shao, Y.; Li, B.; Wu, Y.; Zhang, W.; Zhou, Y.; Yu, Z.; Lu, L.; Wang, X.; et al. Solid-phase microextraction integrated nanobiosensors for the serial detection of cytoplasmic dopamine in a single living cell. Biosens. Bioelectron. 2021, 175, 112915. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y. Development of fluorescent reagent based on ligand exchange reaction for the highly sensitive and selective detection of dopamine in the serum. Sensors 2019, 19, 12. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, P.A.; Lee, J.-S. Recent advances in optical detection of dopamine using nanomaterials. Microchim. Acta 2017, 184, 1239–1266. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, Y.; Lv, C.; Liu, W.; Zhang, Z.; Peng, X. Copper-based metal–organic xerogels on paper for chemiluminescence detection of dopamine. Anal. Methods 2020, 12, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Salehnia, F.; Beigi, S.M.; Aghabalazadeh, S.; Hosseini, M.; Ganjali, M.R. Enhanced peroxidase-like activity of platinum nanoparticles decorated on nickel- and nitrogen-doped graphene nanotubes: Colorimetric detection of glucose. Microchim Acta 2019, 186, 1–9. [Google Scholar] [CrossRef]
- Jiao, A.; Xu, L.; Tian, Y.; Cui, Q.; Liu, X.; Chen, M. Cu2O nanocubes-grafted highly dense Au nanoparticles with modulated electronic structures for improving peroxidase catalytic performances. Talanta 2021, 225, 121990. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Mizani, F.; Hosseini, M.; Keihan, A.H.; Ganjali, M.R. Detection of hydrogen peroxide and glucose by using Tb2(MoO4)3 nanoplates as peroxidase mimics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 186, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Dai, Z.; Hu, X.; Chen, K.; Gao, C.; Zheng, X.; Yu, Y. Synthesis of PB@FePt hybrid nanoparticles with peroxidase-mimicking activity for colorimetric determination of hydrogen peroxide in living cells. Anal. Methods 2019, 11, 677–683. [Google Scholar] [CrossRef]
- Dong, L.; Li, R.; Wang, L.; Lan, X.; Sun, H.; Zhao, Y.; Wang, L. Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose. Int. J. Biol. Macromol. 2021, 172, 289–298. [Google Scholar] [CrossRef]
- Ivanova, M.N.; Grayfer, E.D.; Plotnikova, E.E.; Kibis, L.S.; Darabdhara, G.; Boruah, P.K.; Das, M.R.; Fedorov, V.E. Pt-decorated boron nitride nanosheets as artificial nanozyme for detection of dopamine. ACS Appl. Mater. Interfaces 2019, 11, 22102–22112. [Google Scholar] [CrossRef]
- Fan, L.; Ji, X.; Lin, G.; Liu, K.; Chen, S.; Ma, G.; Xue, W.; Zhang, X.; Wang, L. Green synthesis of stable platinum nanoclusters with enhanced peroxidase-like activity for sensitive detection of glucose and glutathione. Microchem. J. 2021, 166, 106202. [Google Scholar] [CrossRef]
- Wu, R.; Chong, Y.; Fang, G.; Jiang, X.; Pan, Y.; Chen, C.; Yin, J.-J.; Ge, C. Synthesis of Pt hollow nanodendrites with enhanced peroxidase-like activity against bacterial infections: Implication for wound healing. Adv. Funct. Mater. 2018, 28, 1801484.1–1801484.11. [Google Scholar]
- Kang, Z.W.; Kankala, R.K.; Chen, B.Q.; Fu, C.P.; Wang, S.B.; Chen, A.Z. Supercritical fluid-assisted fabrication of manganese (III) oxide hollow nanozymes mediated by polymer nanoreactors for efficient glucose sensing characteristics. ACS Appl. Mater. Interfaces 2019, 11, 28781–28790. [Google Scholar] [CrossRef]
- Cong, C.; Bian, K.; Zhang, X.; Luo, L.; Li, L.; He, H.; Li, C.; Zhao, Q.; Wang, S.; Hao, Z.; et al. Sensitive measurement of tumor markers somatostatin receptors using an octreotide-directed Pt nano-flakes driven electrochemical sensor. Talanta 2020, 208, 120286. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Meng, Z.; Zhang, Y.; Cai, S.; Zhang, Z.; Li, C.; Shang, L.; Shen, Y. Ultrasmall Pt nanoclusters as robust peroxidase mimics for colorimetric detection of glucose in human serum. ACS Appl. Mater. Interfaces 2017, 9, 10027–10033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Yang, J.; Jiang, Y.; He, N. Peroxidase-like activity of mesoporous silica encapsulated Pt nanoparticle and its application in colorimetric immunoassay. Anal. Chim. Acta 2015, 862, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Shim, J.; Kim, K.; Oh, B.T. Prunus x yedoensis tree gum mediated synthesis of platinum nanoparticles with antifungal activity against phytopathogens. Mater. Lett. 2016, 172, 61–65. [Google Scholar] [CrossRef]
- Syed, A.; Ahmad, A. Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surfaces B 2012, 97, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.H.; Cho, J.Y.; Bin Sadiq, N.; Kim, J.-C.; Lee, B.; Hamayun, M.; Lee, T.S.; Kim, H.S.; Park, S.H.; Nho, C.W.; et al. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021, 335, 127645. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Ju, P.; Wang, Z.; Zhang, Y.; Zhai, X.F.; Jiang, F.H.; Sun, C.J. Colorimetric detection of H2O2 based on the enhanced peroxidase mimetic activity of nanoparticles decorated Ce2(WO4)3 nanosheets. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 10. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Feng, Y.; Wang, W.; Jia, L.; Zhang, J. Characterization and hepatoprotections of Ganoderma lucidum Polysaccharides against multiple organ dysfunction syndrome in mice. Oxid. Med. Cell. Longev. 2021, 2021, 9703682. [Google Scholar] [CrossRef] [PubMed]
- Darija, C.R.; Eljko, K.; MaA, K.H.I. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar]
- Zhang, H.; Cui, S.W.; Nie, S.-P.; Chen, Y.; Wang, Y.-X.; Xie, M.-Y. Identification of pivotal components on the antioxidant activity of polysaccharide extract from Ganoderma atrum. Bioact. Carbohydr. Diet. Fibre 2016, 7, 9–18. [Google Scholar] [CrossRef]
- Pan, D.; Wang, L.; Chen, C.; Teng, B.; Wang, C.; Xu, Z.; Hu, B.; Zhou, P. Structure characterization of a novel neutral polysaccharide isolated from Ganoderma lucidum fruiting bodies. Food Chem. 2012, 135, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Kim, Y.G.; Kim, D.; Hyeon, T. Inorganic nanoparticles with enzyme-mimetic activities for biomedical applications. Coord. Chem. Rev. 2020, 403, 213092. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B 2018, 254, 690–700. [Google Scholar] [CrossRef]
- Cui, T.; Li, S.; Chen, S.; Liang, Y.; Sun, H.; Wang, L. “Stealth” dendrimers with encapsulation of indocyanine green for photothermal and photodynamic therapy of cancer. Int. J. Pharm. 2021, 600, 120502. [Google Scholar] [CrossRef] [PubMed]
- Choleva, T.G.; Gatselou, V.A.; Tsogas, G.Z.; Giokas, D.L. Intrinsic peroxidase-like activity of rhodium nanoparticles, and their application to the colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2018, 185, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, J.; Wu, P. Photo-modulated nanozymes for biosensing and biomedical applications. Anal. Methods 2019, 11, 5081–5088. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Jin, S.; Wu, C.; Ye, Z.; Ying, Y. Designed inorganic nanomaterials for intrinsic peroxidase mimics: A review. Sensor. Actuat. B Chem. 2019, 283, 18–34. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Zhou, Q.; Hu, L.; Fu, W.; Wang, Y. Peroxidase-like activity of metal-organic framework [Cu(PDA)(DMF)] and Its application for colorimetric detection of dopamine. ACS Appl. Mater. Interfaces 2019, 11, 44466–44473. [Google Scholar] [CrossRef] [PubMed]
- Aparna, T.K.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloys Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Josypcuk, O.; Barek, J.; Josypcuk, B. Amperometric determination of catecholamines by enzymatic biosensors in flow systems. Electroanalysis 2018, 30, 1163–1171. [Google Scholar] [CrossRef]
- Minta, D.; Gonzalez, Z.; Wiench, P.; Gryglewicz, S.; Gryglewicz, G. N-doped reduced graphene oxide/gold nanoparticles composite as an improved sensing platform for simultaneous detection of dopamine, ascorbic acid, and uric acid. Sensors 2020, 20, 4427. [Google Scholar] [CrossRef]
- Zhao, X.; He, D.; Wang, Y.; Fu, C. Facile fabrication of tungsten disulfide quantum dots (WS2 QDs) as effective probes for fluorescence detection of dopamine (DA). Mater. Chem. Phys. 2018, 207, 130–134. [Google Scholar] [CrossRef]
- Pan, J.; Miao, C.; Chen, Y.; Ye, J.; Weng, S. Facile fluorescence dopamine detection strategy based on acid phosphatase (ACP) enzymatic oxidation dopamine to polydopamine. Chem. Pharm. Bull. 2020, 68, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ding, Y.-N.; Bian, B.; Li, L.; Li, R.; Zhang, X.; Liu, Z.; Zhang, X.; Fan, G.; Liu, Q. Rapid colorimetric determination of dopamine based on the inhibition of the peroxidase mimicking activity of platinum loaded CoSn(OH)6 nanocubes. Microchim. Acta 2019, 186, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, J.; Jing, P.; Yao, W.; Gao, X.; Yu, L. Preparation of a hydroxypropyl Ganoderma lucidum polysaccharide and its physicochemical properties. Food Chem. 2010, 122, 965–971. [Google Scholar] [CrossRef]











| Catalyst | Substrate | Km (mM) | Vmax (10−8 M s−1) | Reference |
|---|---|---|---|---|
| Pt600-GLP NCs | TMB | 0.17 | 5.04 | this work |
| Pt600-GLP NCs | H2O2 | 2.06 | 7.51 | |
| HRP | TMB | 0.434 | 10.00 | [33] |
| HRP | H2O2 | 3.70 | 8.71 |
| Materials | Linear Range (μM) | Detection Limit (μM) | Method | Reference |
|---|---|---|---|---|
| Au-Cu2O/rGO | 10–90 | 3.9 | Electrochemistry | [36] |
| Lac-SBA15 | 4–500 | 4.05 | Electrochemistry | [37] |
| GCE/N-rGO-Au | 3–100 | 2.4 | Electrochemistry | [38] |
| WS2 QDs | 3–50 | 3.3 | Fluorescence | [39] |
| ACP-CDs | 3–20 | 1 | Fluorescence | [40] |
| Pt/hBNNSs-5 | 2–55 | 0.76 | Colorimetry | [11] |
| Pt/CoSn(OH)6 | 5–60 | 0.76 | Colorimetry | [41] |
| Pt600-GLP NCs | 1–100 | 0.66 | Colorimetry | this work |
| Sample | Added Amount (μM) | Found Amount (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Serum | 15 | 14.85 | 99.03 | 2.11 |
| Serum | 30 | 28.99 | 96.66 | 1.28 |
| Serum | 45 | 44.46 | 98.80 | 3.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, X.; Han, Y.; Zhang, J.; Zhang, J.; Lin, W.; Liu, Z.; Wang, L. Peroxidase-Like Platinum Clusters Synthesized by Ganoderma lucidum Polysaccharide for Sensitively Colorimetric Detection of Dopamine. Molecules 2021, 26, 2738. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092738
Lai X, Han Y, Zhang J, Zhang J, Lin W, Liu Z, Wang L. Peroxidase-Like Platinum Clusters Synthesized by Ganoderma lucidum Polysaccharide for Sensitively Colorimetric Detection of Dopamine. Molecules. 2021; 26(9):2738. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092738
Chicago/Turabian StyleLai, Xiang, Yu Han, Jie Zhang, Jinyu Zhang, Weifeng Lin, Zhiwei Liu, and Longgang Wang. 2021. "Peroxidase-Like Platinum Clusters Synthesized by Ganoderma lucidum Polysaccharide for Sensitively Colorimetric Detection of Dopamine" Molecules 26, no. 9: 2738. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26092738