Profiles of Essential Oils and Correlations with Phenolic Acids and Primary Metabolites in Flower Buds of Magnolia heptapeta and Magnolia denudata var. purpurascens
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition and Contents of Essential Oils
2.2. Quantification of Phenolic Acids by HPLC
2.3. Metabolic Profiling of Magnolia Flower Buds by PCA
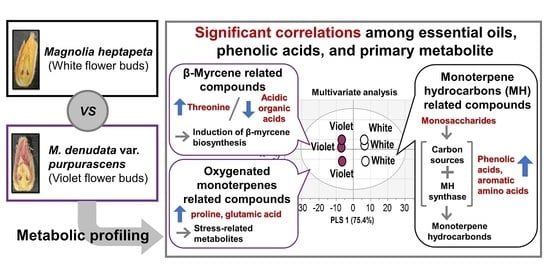
2.4. Correlations among Essential Oils, Phenolic Acids, and Hydrophilic Compounds
2.5. Classification of Magnolia Flower Buds Based On PLS-DA
3. Materials and Methods
3.1. Preparation of Plant Materials
3.2. Essential Oil Extraction
3.3. Chemical Composition Analysis of Essential Oil
3.4. Phenolic Acid Extraction and Analysis
3.5. Low-Molecular-Weight Hydrophilic Compound Extraction and Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Farag, M.A.; Al-Mahdy, D.A. Comparative Study of the Chemical Composition and Biological Activities of Magnolia Grandiflora and Magnolia virginiana Flower Essential Oils. Nat. Prod. Res. 2013, 27, 1091–1097. [Google Scholar] [CrossRef]
- Jeong, J.-G. A Herbological Study on the Plants of Magnoliaceae in Korea. Kor. J. Herbol. 2018, 33, 57–63. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Yoon, J.I.; Kang, S.C. Antioxidant and Antidermatophytic Activities of Essential Oil and Extracts of Magnolia liliflora Desr. Food Chem. Toxicol. 2009, 47, 2606–2612. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, D.; Dong, Y.; Chen, X.; Wang, X. The Variation of Oxidative Stability and Sensory Attributes of Sunflower Oil Induced by Essential Oil from Magnolia liliflora Desr. during High-temperature Storage. Int. J. Food Sci. Tech. 2021, 56, 3355–3366. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.-S.; Eom, S.J.; Kim, K.; Paik, H.-D. Antioxidative and Anticanceric Activities of Magnolia (Magnolia denudata) Flower Petal Extract Fermented by Pediococcus acidilactici KCCM 11614. Molecules 2015, 20, 12154–12165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.; Bai, M.; Ye, W.; Wang, Y.; Wu, H. Variations in Volatile Oil Yield and Composition of “Xin-Yi”(Magnolia biondii Pamp. Flower Buds) at Different Growth Stages. J. Oleo Sci. 2018, 67, 779–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowndhararajan, K.; Cho, H.; Yu, B.; Kim, S. Comparison of Essential Oil Compositions of Fresh and Dried Fruits of Magnolia Kobus DC. J. Appl. Pharm. Sci 2016, 6, 146–149. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.; Seo, G.-U.; Yuk, H.-G.; Lee, S. Antioxidant and Tyrosinase Inhibitory Activities of Methanol Extracts from Magnolia denudata and Magnolia denudata var. Purpurascens Flowers. Food Res. Int. 2012, 47, 197–200. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Ma, Q.; Wei, R.; Zhang, X.; Sang, Z.; Dong, J. Structural Elucidation and Neuroprotective Activities of Lignans from the Flower Buds of Magnolia biondii Pamp. Z. Naturforsch. C 2021, 76, 147–152. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Maggi, F. Chemical Composition, Antioxidant Activity and Cytotoxicity on Tumour Cells of the Essential Oil from Flowers of Magnolia Grandiflora Cultivated in Iran. Nat. Prod. Res. 2017, 31, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Souri, E.; Amin, G.; Farsam, H.; Barazandeh, T.M. Screening of Antioxidant Activity and Phenolic Content of 24 Medicinal Plant Extracts. DARU 2008, 16, 83–87. [Google Scholar]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of some Secondary Compounds Derived from the Primary Metabolism for their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [Green Version]
- Meng-Ying, L.V.; Jian-Bo, S.; Min, W.; Hong-Yan, F.; Zhang, Z.; Feng-Guo, X.U. Comparative Analysis of Volatile Oils in the Stems and Roots of Ephedra sinica via GC-MS-Based Plant Metabolomics. Chin. J. Nat. Med. 2016, 14, 133–140. [Google Scholar] [CrossRef]
- Xiang, Z.; Wang, X.; Gao, H.; Zhang, M.; Zeng, S. GC-MS and HPLC Metabolic Profiling Studies of Curcuma wenyujin Rhizomes obtained at Different Harvest Times. Anal. Lett. 2012, 45, 1–14. [Google Scholar] [CrossRef]
- Rai, A.; Saito, K.; Yamazaki, M. Integrated Omics Analysis of Specialized Metabolism in Medicinal Plants. Plant J. 2017, 90, 764–787. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.-Y.; Lim, S.-H.; Yeo, Y.; Cho, H.S.; Ha, S.-H. Comparative Metabolic Profiling of Pigmented Rice (Oryza Sativa L.) Cultivars Reveals Primary Metabolites are Correlated with Secondary Metabolites. J. Cereal Sci. 2013, 57, 14–20. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lim, S.-H.; Ha, S.-H.; Yeo, Y.; Park, W.T.; Kwon, D.Y.; Park, S.U.; Kim, J.K. Metabolite Profiling Approach Reveals the Interface of Primary and Secondary Metabolism in Colored Cauliflowers (Brassica Oleracea L. ssp. Botrytis). J. Agric. Food Chem. 2013, 61, 6999–7007. [Google Scholar] [CrossRef]
- Park, C.H.; Park, S.; Lee, S.Y.; Kim, J.K.; Park, S.U. Analysis of Metabolites in White Flowers of Magnolia denudata Desr. and Violet Flowers of Magnolia liliiflora Desr. Molecules 2018, 23, 1558. [Google Scholar] [CrossRef] [Green Version]
- Zrira, S.; Bessiere, J.M.; Menut, C.; Elamrani, A.; Benjilali, B. Chemical Composition of the Essential Oil of Nine Eucalyptus Species Growing in Morocco. Flavour fragr. J. 2004, 19, 172–175. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What are the Potential Health Benefits of this Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 400. [Google Scholar] [CrossRef]
- Schühly, W.; Ross, S.A.; Mehmedic, Z.; Fischer, N.H. Essential Oil Analysis of the Follicles of Four North American Magnolia Species. Nat. Prod. Commun. 2008, 3, 1934578X0800300715. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Li, R.; Jiang, Z.; Wang, Y.; Tan, J.; Tang, S.; Zhang, Y. Screening and Evaluation of Radical Scavenging Active Compounds in the Essential Oil from Magnolia biondii Pamp by Electronic Nose Coupled with Chemical Methodology. Ind. Crops Prod. 2020, 144, 112060. [Google Scholar] [CrossRef]
- Ebadi, M.; Mollaei, S.; Khurizadeh, S. Evaluation of Volatile and Phenolic Compounds, and Antioxidant Activity of Different Parts of Ferulago angulata (Schlecht.) Boiss. Ind. Crops Prod. 2019, 140, 111589. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, V.S.; Trifan, A.; Miron, A. Antigenotoxic potential of some dietary non-phenolic phytochemicals. In Studies in Natural Products Chemistry; ur-Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 60, pp. 223–297. [Google Scholar] [CrossRef]
- Kurade, N.P.; Jaitak, V.; Kaul, V.K.; Sharma, O.P. Chemical Composition and Antibacterial Activity of Essential Oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm. Biol. 2010, 48, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Acharya, B.; Chaijaroenkul, W.; Na-Bangchang, K. Therapeutic Potential and Pharmacological Activities of β-eudesmol. Chem. Biol. Drug Des. 2021, 97, 984–996. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Chapter Three—Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 67–134. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Cen, Z.; Fu, Y.; Zhu, X.; He, H.; Kong, D.; Wu, H. The Variation in Essential Oils Composition, Phenolic Acids and Flavonoids is Correlated with Changes in Antioxidant Activity during Cinnamomum loureirii Bark Growth. Arab. J. Chem. 2021, 14, 103249. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M. Variation in Essential Oil Composition, Bioactive Compounds, Anatomical and Antioxidant Activity of Achillea Aucheri, an Endemic Species of Iran, at Different Phenological Stages. Chem. Biodivers. 2018, 15, e1800319. [Google Scholar] [CrossRef]
- Hazrati, S.; Mollaei, S.; Rabbi Angourani, H.; Hosseini, S.J.; Sedaghat, M.; Nicola, S. How do Essential Oil Composition and Phenolic Acid Profile of Heracleum persicum Fluctuate at Different Phenological Stages? Food Sci. Nutr. 2020, 8, 6192–6206. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Patel, D.K. The Beneficial Role of Rutin, a Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 457–479. [Google Scholar] [CrossRef]
- Guo, L.; Wang, S.; Zhang, J.; Yang, G.; Zhao, M.; Ma, W.; Zhang, X.; Li, X.; Han, B.; Chen, N. Effects of Ecological Factors on Secondary Metabolites and Inorganic Elements of Scutellaria baicalensis and Analysis of Geoherblism. Sci. China Life Sci. 2013, 56, 1047–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, V.; Farah, A. Chlorogenic Acids and Related Compounds in Medicinal Plants and Infusions. Food Chem. 2009, 113, 1370–1376. [Google Scholar] [CrossRef]
- Hyeon, H.; Xu, J.L.; Kim, J.K.; Choi, Y. Comparative Metabolic Profiling of Cultivated and Wild Black Soybeans Reveals Distinct Metabolic Alterations Associated with their Domestication. Food Res. Int. 2020, 134, 109290. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Hyeon, H.; Park, N.I.; Yi, T.G.; Lim, S.-H.; Park, S.-Y.; Ha, S.-H.; Kim, J.K. A High-Throughput Platform for Interpretation of Metabolite Profile Data from Pepper (Capsicum) Fruits of 13 Phenotypes Associated with Different Fruit Maturity States. Food Chem. 2020, 331, 127286. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Tenori, L.; Cascante, M.; Carulla, P.R.; Dos Santos, V.A.; Saccenti, E. From Correlation to Causation: Analysis of Metabolomics Data using Systems Biology Approaches. Metabolomics 2018, 14, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saccenti, E.; Timmerman, M.E. Approaches to Sample Size Determination for Multivariate Data: Applications to PCA and PLS-DA of Omics Data. J. Proteome Res. 2016, 15, 2379–2393. [Google Scholar] [CrossRef]
- Hyeon, H.; Min, C.W.; Moon, K.; Cha, J.; Gupta, R.; Park, S.U.; Kim, S.T.; Kim, J.K. Metabolic Profiling-Based Evaluation of the Fermentative Behavior of Aspergillus oryzae and Bacillus Subtilis for Soybean Residues Treated at Different Temperatures. Foods 2020, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So You Think You can PLS-DA? BMC Bioinform. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Pazouki, L.; Niinemets, Ü. Multi-Substrate Terpene Synthases: Their Occurrence and Physiological Significance. Front. Plant Sci. 2016, 7, 1019. [Google Scholar] [CrossRef] [Green Version]
- Korman, T.P.; Opgenorth, P.H.; Bowie, J.U. A Synthetic Biochemistry Platform for Cell Free Production of Monoterpenes from Glucose. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Trapp, S.; Croteau, R. Defensive Resin Biosynthesis in Conifers. Annu. Rev. Plant Biol. 2001, 52, 689–724. [Google Scholar] [CrossRef]
- El Tamer, M.K. Molecular Regulation of Plant Monoterpene Biosynthesis in Relation to Fragrance. 2002. Available online: https://edepot.wur.nl/121357 (accessed on 5 December 2021).
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Dudareva, N.; Martin, D.; Kish, C.M.; Kolosova, N.; Gorenstein, N.; Fäldt, J.; Miller, B.; Bohlmann, J. (E)-β-Ocimene and Myrcene Synthase Genes of Floral Scent Biosynthesis in Snapdragon: Function and Expression of Three Terpene Synthase Genes of a New Terpene Synthase Subfamily. Plant Cell 2003, 15, 1227–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought Stress Adaptation Modulates Plant Secondary Metabolite Production in Salvia Dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Trovato, M.; Forlani, G.; Signorelli, S.; Funck, D. Proline metabolism and its functions in development and stress tolerance. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 41–72. [Google Scholar] [CrossRef]
- Shimizu, K. Main metabolism. In Bacterial Cellular Metabolic Systems; Woodhead Publ. Co.: Oxford, UK, 2013; pp. 1–54. [Google Scholar]
- Nagegowda, D.A. Plant Volatile Terpenoid Metabolism: Biosynthetic Genes, Transcriptional Regulation and Subcellular Compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdizadeh, L.; Ghasemi Pirbalouti, A.; Moghaddam, M. Storage Stability of Essential Oil of Cumin (Cuminum Cyminum L.) as a Function of Temperature. Int. J. Food Prop. 2017, 20, 1742–1750. [Google Scholar] [CrossRef]
- Ramírez, J.; Andrade, M.D.; Vidari, G.; Gilardoni, G. Essential Oil and Major Non-Volatile Secondary Metabolites from the Leaves of Amazonian Piper subscutatum. Plants 2021, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Maggi, F.; Tirillini, B. Essential Oil Composition of Hypericum richeri Vill. from Italy. Flavour Fragr. J. 2005, 20, 295–298. [Google Scholar] [CrossRef]
- Rahimi, M.R.; Zamani, R.; Sadeghi, H.; Tayebi, A.R. An Experimental Study of Different Drying Methods on the Quality and Quantity Essential Oil of Myrtus Communis L. Leaves. J. Essent. Oil-Bear. Plants 2015, 18, 1395–1405. [Google Scholar] [CrossRef]
- Gamal El-Din, M.I.; Youssef, F.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Comparative Analysis of Volatile Constituents of Pachira Aquatica Aubl. and Pachira Glabra Pasq., their Anti-Mycobacterial and Anti-Helicobacter Pylori Activities and their Metabolic Discrimination using Chemometrics. J. Essent. Oil-Bear. Plants 2018, 21, 1550–1567. [Google Scholar] [CrossRef]
- Yeo, H.J.; Park, C.H.; Park, Y.E.; Hyeon, H.; Kim, J.K.; Lee, S.Y.; Park, S.U. Metabolic Profiling and Antioxidant Activity during Flower Development in Agastache Rugosa. Physiol. Mol. Biol. Plants 2021, 27, 445–455. [Google Scholar] [CrossRef]




| NO. | Type | Compounds | RT | RI | Relative Content (%) | |
|---|---|---|---|---|---|---|
| White | Violet | |||||
| 1 | MH | α-Thujene | 8.028 | 925 | 0.552 ± 0.003 | 0.498 ± 0.005 |
| 2 | MH | α-Pinene | 8.234 | 930 | 6.328 ± 0.019 | 4.828 ± 0.087 |
| 3 | MH | Camphene | 8.787 | 944 | 0.406 ± 0.009 | 0.248 ± 0.003 |
| 4 | MH | Sabinene | 9.787 | 971 | 15.789 ± 0.055 | 14.58 ± 0.334 |
| 5 | MH | β-Pinene | 9.869 | 973 | 12.921 ± 0.052 | 7.668 ± 0.202 |
| 6 | MH | β-Myrcene | 10.581 | 992 | 9.936 ± 0.052 | 17.194 ± 0.143 |
| 7 | MH | α-Phellandrene | 11.051 | 1003 | 0.444 ± 0.002 | 0.363 ± 0.004 |
| 8 | MH | δ-3-Carene | 11.292 | 1009 | 0.196 ± 0.002 | 0.107 ± 0.002 |
| 9 | MH | α-Terpinene | 11.575 | 1015 | 1.788 ± 0.011 | 1.254 ± 0.003 |
| 10 | MH | Limonene | 12.086 | 1027 | 3.811 ± 0.006 | 2.948 ± 0.058 |
| 11 | MO | 1,8-Cineole | 12.192 | 1029 | 7.589 ± 0.007 | 11.606 ± 0.193 |
| 12 | MH | (Z)-β-Ocimene | 13.039 | 1048 | 0.231 ± 0.004 | 0.240 ± 0.005 |
| 13 | MH | γ-Terpinene | 13.439 | 1057 | 4.644 ± 0.014 | 2.881 ± 0.029 |
| 14 | MH | α-Terpinolene | 14.757 | 1087 | 0.889 ± 0.006 | 0.818 ± 0.007 |
| 15 | MO | Camphor | 17.286 | 1143 | 0.238 ± 0.001 | 0.354 ± 0.004 |
| 16 | MO | 3-Cyclohexen-1-ol | 18.810 | 1147 | 0.876 ± 0.004 | 0.954 ± 0.002 |
| 17 | MO | 3-Cyclohexene-1-methanol | 19.451 | 1168 | 1.499 ± 0.009 | 1.613 ± 0.023 |
| 18 | MO | Borneol acetate | 23.715 | 1286 | 0.440 ± 0.003 | 0.295 ± 0.004 |
| 19 | SH | α-Copaene | 27.485 | 1374 | 0.114 ± 0.001 | 0.100 ± 0.003 |
| 20 | SH | β-Elemene | 28.209 | 1391 | 0.333 ± 0.009 | 0.673 ± 0.008 |
| 21 | SH | β-Caryophyllene | 29.274 | 1417 | 2.610 ± 0.042 | 2.031 ± 0.038 |
| 22 | SH | α-Humulene | 30.668 | 1451 | 0.660 ± 0.009 | 0.562 ± 0.018 |
| 23 | SH | Germacrene D | 31.809 | 1479 | 7.729 ± 0.037 | 7.986 ± 0.234 |
| 24 | SH | α-Muurolene | 32.638 | 1500 | 0.399 ± 0.014 | 0.665 ± 0.020 |
| 25 | SH | δ-Cadinene | 33.544 | 1523 | 2.682 ± 0.049 | 2.495 ± 0.089 |
| 26 | SO | Elemol | 34.573 | 1550 | 1.042 ± 0.004 | 0.193 ± 0.005 |
| 27 | SO | trans-Nerolidol | 35.173 | 1566 | 0.491 ± 0.010 | 0.586 ± 0.015 |
| 28 | SO | β-Eudesmol | 38.302 | 1650 | 3.658 ± 0.023 | 0.908 ± 0.023 |
| 29 | SO | α-Eudesmol | 38.420 | 1653 | 2.039 ± 0.032 | 0.430 ± 0.032 |
| 30 | SO | α-Cadinol | 38.508 | 1655 | 2.905 ± 0.017 | 2.426 ± 0.054 |
| Monoterpene hydrocarbons (MH) | 57.936 ± 0.118 | 53.627 ± 0.582 | ||||
| Oxygenated monoterpenes (MO) | 10.642 ± 0.012 | 14.823 ± 0.162 | ||||
| Sesquiterpene hydrocarbons (SH) | 14.528 ± 0.122 | 14.513 ± 0.403 | ||||
| Oxygenated sesquiterpenes (SO) | 10.136 ± 0.043 | 4.543 ± 0.111 | ||||
| Total | 93.24 | 87.51 | ||||
| NO. | Name (mg/g Dry Weight) | Formula | White | Violet |
|---|---|---|---|---|
| 1 | Chlorogenic acid | C16H18O9 | 3.548 ± 0.285 | 4.372 ± 0.075 |
| 2 | Caffeic acid | C9H8O4 | 0.186 ± 0.008 | 0.356 ± 0.011 |
| 3 | Coumaric acid | C9H8O3 | 0.117 ± 0.003 | 0.065 ± 0.004 |
| 4 | Rutin | C27H30O16 | 69.136 ± 4.625 | 43.200 ± 2.763 |
| 5 | Ferulic acid | C10H10O4 | 0.132 ± 0.006 | 0.156 ± 0.001 |
| 6 | Cinnamic acid | C9H8O2 | 0.084 ± 0.003 | 0.018 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyeon, H.; Hyun, H.B.; Go, B.; Kim, S.C.; Jung, Y.-H.; Ham, Y.-M. Profiles of Essential Oils and Correlations with Phenolic Acids and Primary Metabolites in Flower Buds of Magnolia heptapeta and Magnolia denudata var. purpurascens. Molecules 2022, 27, 221. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27010221
Hyeon H, Hyun HB, Go B, Kim SC, Jung Y-H, Ham Y-M. Profiles of Essential Oils and Correlations with Phenolic Acids and Primary Metabolites in Flower Buds of Magnolia heptapeta and Magnolia denudata var. purpurascens. Molecules. 2022; 27(1):221. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27010221
Chicago/Turabian StyleHyeon, Hyejin, Ho Bong Hyun, Boram Go, Sung Chun Kim, Yong-Hwan Jung, and Young-Min Ham. 2022. "Profiles of Essential Oils and Correlations with Phenolic Acids and Primary Metabolites in Flower Buds of Magnolia heptapeta and Magnolia denudata var. purpurascens" Molecules 27, no. 1: 221. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27010221