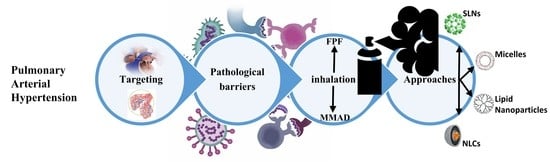
An Update on Advancements and Challenges in Inhalational Drug Delivery for Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. The Advantages of Inhaled Medication Delivery
3. Adverse Functional Changes Associated with PAH
4. Particle Deposition at Pulmonary vasculature and Inhaled performance
4.1. Duration of action
4.2. Scarcity of Dosing Accuracy
4.3. Aerosolized Particle Size
4.3.1. Mass Median Aerodynamic Diameter
4.3.2. Fine Particle Fraction
4.4. Devices for Inhaled Administration
4.4.1. Nebulizers
Jet Nebulizers
Ultrasonic Nebulizers
Mesh Nebulizers
4.4.2. Pressurized Metered Dose Inhalers (pMDIs)
4.4.3. Dry Powder Inhalers (DPIs)
4.4.4. Iloprost (Ventavis)
4.4.5. Treprostinil (Tyvaso)
5. Novel Formulation Approaches
5.1. Lipid Nanoparticles
5.1.1. Solid Lipid Nanoparticles
5.1.2. Nanostructured Lipid carriers
5.2. The Micelles
5.3. Particle Size and Lung Retention
6. Conclusions
7. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine | DSPE |
| 1,2-dioleoyl-sn-glycero-3-phosphocholine | DOPC |
| 1,2-Distearoyl-sn-glycero-3-phosphocholine | DSPC |
| alveolar epithelial type I | AEC I |
| alveolar epithelial type II | AEC II |
| alveolar macrophages | AMs |
| AMP kinase | AMPK |
| Bone morphogenetic protein receptor | BMPR2 |
| Bovine serum albumin | BSA |
| CARSKNKDC | CAR |
| danger-associated molecular pattern | DAMP |
| dioleoylphosphatidylglycerol | DOTAP |
| dipalmitoylphosphatidylcholine | DPPC |
| Emitted dose | ED |
| Endothelial-mesenchymal transition | EndMT |
| fine particle fraction | FPF |
| High Mobility Group-box | HMGB1 |
| hyaluronic acid | HA |
| Hydroxypropyl Methylcellulose | HPMC |
| Hypoxia-inducible factor 1-alpha | HIF-1α |
| Interleukins | IL |
| layer by-layer assembly | LbL |
| lipid nanoparticles | LN |
| Magnetic Nanoparticles | MNPs |
| mass median aerodynamic diameter | MMAD |
| mean pulmonary arterial pressure | mPAP |
| Monocrotaline model | MCT |
| Monocyte Chemoattractant Protein | (MCP)-1 |
| N,N′-Dicyclohexylcarbodiimide | DCC |
| Nanoparticles | NPs |
| Nanostructured Lipid carriers | NLC |
| N-Hydroxysuccinimide | NHS |
| Nitric Oxide | NO |
| oxidative phosphorylation | OXPHOS |
| pattern recognition receptors | PRRs |
| PEGylated derivative of 1,2-distearoyl-sn-glycero-3-PE | DSPE-mPEG2000 |
| Peroxisome-proliferator-activated-receptor-gamma | PPAR-γ |
| phosphodiesterase 5 | PDE5 |
| photon correlation spectroscopy | PCS |
| platelet-derived growth factor | PDGF |
| pluronic® mixed micelles | PMM |
| Poly (ADP)-ribose-1 | PARP-1 |
| poly(ε-caprolactone) | PCL |
| polydispersity index | PDI |
| Polyethylene glycol | PEG |
| poly-lactic-co-glycolic acid | PLGA |
| Polyvinylpyrrolidone | PVP |
| Pulmonary arterial endothelial | PAECs |
| Pulmonary Arterial Hypertension | PAH |
| Pulmonary arterial smooth muscle cells | PASMCs |
| Pulmonary Hypertension | PH |
| pulmonary vascular resistance | PVR |
| pyruvate dehydrogenase | PDH |
| pyruvate dehydrogenase kinase | PDK |
| Reactive Oxygen Species | ROS |
| Right ventricle | RV |
| Self-Nanoemulsifying Drug Delivery System | SNEDDS |
| Superoxide dismutase | SOD |
| tissue-resident dendritic cells | DCs |
| Trifluoroacetic acid | TFA |
| tumor necrosis factor | TNF |
| Vasoactive intestinal peptide | VIP |
References
- Badesch, D.B.; Champion, H.C.; Sanchez, M.A.G.; Hoeper, M.M.; Loyd, J.E.; Manes, A.; McGoon, M.; Naeije, R.; Olschewski, H.; Oudiz, R.J.; et al. Diagnosis and Assessment of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2009, 54, S55–S66. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Giannoulatou, E.; Celermajer, D.; Humbert, M. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Archer, S.L. The right ventricle in pulmonary arterial hypertension: Disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ. Res. 2014, 115, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.J. Pulmonary Hypertension. JAMA 2012, 308, 1366. Available online: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2012.12347 (accessed on 30 November 2021). [CrossRef] [PubMed]
- McLaughlin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the Ameri-can Heart Association Developed in Collaboration with the American College of Chest Physicians; American Thoracic So-ciety, Inc.; and the Pulmonary Hypertension Association. Circulation 2009, 119, 1573–1619. [Google Scholar]
- Taichman, D.B.; Ornelas, J.; Chung, L.; Klinger, J.R.; Lewis, S.; Mandel, J.; Palevsky, H.I.; Rich, S.; Sood, N.; Rosenzweig, E.B.; et al. Pharmacologic Therapy for Pulmonary Arterial Hypertension in Adults. Chest 2014, 146, 449–475. [Google Scholar] [CrossRef] [Green Version]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Brain, J.D. Inhalation, Deposition, and Fate of Insulin and Other Therapeutic Proteins. Diabetes Technol. Ther. 2007, 9 (Suppl. 1), S4–S15. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Pellosi, D.; D’Angelo, I.; Maiolino, S.; Mitidieri, E.; Bianca, R.D.D.V.; Sorrentino, R.; Quaglia, F.; Ungaro, F. In vitro/in vivo investigation on the potential of Pluronic® mixed micelles for pulmonary drug delivery. Eur. J. Pharm. Biopharm. 2018, 130, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S. Evaluation and classification of pulmonary arterial hypertension. J. Thorac. Dis. 2019, 11, S1789–S1799. Available online: http://jtd.amegroups.com/article/view/31939/22544 (accessed on 1 December 2021). [CrossRef] [PubMed]
- Schermuly, R.T.; Ghofrani, A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Meyrick, B.; Galie, N.; Mooi, W.J.; McMurtry, I.F. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Cell. Mol. Physiol. 2009, 297, L1013–L1032. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Adnot, S.; Archer, S.L.; Dupuis, J.; Lloyd Jones, P.; MacLean, M.R.; McMurtry, I.F.; Stenmark, K.R.; Thistlethwaite, P.A.; Weissmann, N.; et al. Cellular and Molecular Basis of Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2009, 54, S20–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuebler, W.M.; Bonnet, S.; Tabuchi, A. Inflammation and autoimmunity in pulmonary hypertension: Is there a role for endothelial adhesion molecules? (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045893218757596. [Google Scholar] [CrossRef] [Green Version]
- Humbert, M.; Monti, G.; Brenot, F.; Sitbon, O.; Portier, A.; Grangeot-Keros, L.; Duroux, P.; Galanaud, P.; Simonneau, G.; Emilie, D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1995, 151, 1628–1631. [Google Scholar] [CrossRef]
- Soon, E.; Holmes, A.M.; Treacy, C.M.; Doughty, N.J.; Southgate, L.; Machado, R.D.; Trembath, R.C.; Jennings, S.; Barker, L.; Nicklin, P.; et al. Elevated Levels of Inflammatory Cytokines Predict Survival in Idiopathic and Familial Pulmonary Arterial Hypertension. Circulation 2010, 122, 920–927. Available online: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.109.933762 (accessed on 2 December 2021). [CrossRef] [Green Version]
- Steiner, M.K.; Syrkina, O.L.; Kolliputi, N.; Mark, E.J.; Hales, C.A.; Waxman, A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 2009, 104, 236–244. [Google Scholar] [CrossRef]
- Tamura, Y.; Phan, C.; Tu, L.; Le Hiress, M.; Thuillet, R.; Jutant, E.-M.; Fadel, E.; Savale, L.; Huertas, A.; Humbert, M.; et al. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J. Clin. Investig. 2018, 128, 1956–1970. Available online: https://www.jci.org/articles/view/96462 (accessed on 4 December 2021). [CrossRef] [Green Version]
- Diebold, I.; Hennigs, J.K.; Miyagawa, K.; Li, C.G.; Nickel, N.P.; Kaschwich, M.; Cao, A.; Wang, L.; Reddy, S.; Chen, P.-I.; et al. BMPR2 Preserves Mitochondrial Function and DNA during Reoxygenation to Promote Endothelial Cell Survival and Reverse Pulmonary Hypertension. Cell Metab. 2015, 21, 596–608. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S1550413115001138 (accessed on 5 December 2021). [CrossRef] [PubMed] [Green Version]
- Bourgeois, A.; Lambert, C.; Habbout, K.; Ranchoux, B.; Paquet-Marceau, S.; Trinh, I.; Breuils-Bonnet, S.; Paradis, R.; Nadeau, V.; Paulin, R.; et al. FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension. Klin. Wochenschr. 2017, 96, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Pflieger, A.; Vaillancourt, M.; Paulin, R.; Potus, F.; Zervopoulos, S.; Graydon, C.; Courboulin, A.; Breuils-Bonnet, S.; Tremblay, E.; et al. Role for DNA Damage Signaling in Pulmonary Arterial Hypertension. Circulation 2014, 129, 786–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeger, W.; Pullamsetti, S.S. Mechanics and Mechanisms of Pulmonary Hypertension—Conference Summary and Translational Perspectives. Pulm. Circ. 2013, 3, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Firth, A.L.; Yuill, K.H.; Smirnov, S.V. Mitochondria-dependent regulation of Kv currents in rat pulmonary artery smooth muscle cells. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L61–L70. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.-L.; Zhang, Z.-X.; Chen, C.-S.; Cai, C.; Zhao, J.-P.; Wang, X. Effects of Mitochondrial Potassium Channel and Membrane Potential on Hypoxic Human Pulmonary Artery Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2010, 42, 661–666. [Google Scholar] [CrossRef]
- Yang, P.-S.; Kim, D.-H.; Lee, Y.J.; Lee, S.-E.; Kang, W.J.; Chang, H.-J.; Shin, J.-S. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir. Res. 2014, 15, 148. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.J.; Archer, S.L. Emerging Concepts in the Molecular Basis of Pulmonary Arterial Hypertension. Circulation 2015, 131, 1691–1702. Available online: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.114.006979 (accessed on 6 December 2021). [CrossRef] [Green Version]
- Arciniegas, E.; Neves, C.Y.; Carrillo, L.M.; Zambrano, E.A.; Ramírez, R. Endothelial-Mesenchymal Transition Occurs during Embryonic Pulmonary Artery Development. Endothelium 2005, 12, 193–200. [Google Scholar] [CrossRef]
- Good, R.B.; Gilbane, A.J.; Trinder, S.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef]
- Castranova, V.; Rabovsky, J.; Tucker, J.; Miles, P. The alveolar type II epithelial cell: A multifunctional pneumocyte. Toxicol. Appl. Pharmacol. 1988, 93, 472–483. [Google Scholar] [CrossRef]
- Rackley, C.R.; Stripp, B.R. Building and maintaining the epithelium of the lung. J. Clin. Investig. 2012, 122, 2724–2730. [Google Scholar] [CrossRef] [Green Version]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Brownfield, D.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nho, R. Pathological effects of nano-sized particles on the respiratory system. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102242. [Google Scholar] [CrossRef] [PubMed]
- Yacobi, N.R.; Fazllolahi, F.; Kim, Y.H.; Sipos, A.; Borok, Z.; Kim, K.-J.; Crandall, E.D. Nanomaterial interactions with and trafficking across the lung alveolar epithelial barrier: Implications for health effects of air-pollution particles. Air Qual. Atmos. Health 2010, 4, 65–78. [Google Scholar] [CrossRef]
- Paredi, P.; Barnes, P.J. The airway vasculature: Recent advances and clinical implications. Thorax 2009, 64, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Tomic, R.; Antonescu-Turcu, A.; Jacobs, E.R. Development, anatomy, and physiology of the lungs. In Principles of Deglutition A Multidisciplinary Text for Swallowing and its Disorders; Springer: New York, NY, USA, 2013. [Google Scholar]
- Kulkarni, T.; De Andrade, J.; Zhou, Y.; Luckhardt, T.; Thannickal, V.J. Alveolar epithelial disintegrity in pulmonary fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L185–L191. [Google Scholar] [CrossRef] [Green Version]
- Geiser, T. Idiopathic pulmonary fibrosis—A disorder of alveolar wound repair? Swiss Med. Wkly. 2003, 133, 405–411. [Google Scholar]
- Kasper, M.; Barth, K. Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci. Rep. 2017, 37, BSR2017130. [Google Scholar] [CrossRef] [Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.J.; Muralikrishnan, S.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Nanoparticle-induced pulmonary toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Borm, P.J.; Castranova, V.; Gulumian, M. The limits of testing particle-mediated oxidative stress in vitro in predicting diverse pathologies; relevance for testing of nanoparticles. Part. Fibre Toxicol. 2009, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Chen, F.; Mozhi, A.; Zhang, X.; Zhao, Y.; Xue, X.; Hao, Y.; Zhang, X.; Wang, P.C.; Liang, X.-J. Innovative pharmaceutical development based on unique properties of nanoscale deliv-ery formulation. Nanoscale 2013, 15, 8307–8325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, H.; Harder, V.; Ibald-Mulli, A.; Khandoga, A.; Koenig, W.; Krombach, F.; Radykewicz, R.; Stampfl, A.; Thorand, B.; Peters, A. Cardiovascular Effects of Fine and Ultrafine Particles. J. Aerosol Med. 2005, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lucking, A.J.; Lundback, M.; Mills, N.; Faratian, D.; Barath, S.L.; Pourazar, J.; Cassee, F.R.; Donaldson, K.; Boon, N.A.; Badimon, J.J.; et al. Diesel exhaust inhalation increases thrombus formation in man. Eur. Heart J. 2008, 29, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Dishy, V.; Sofowora, G.; Harris, P.A.; Kandcer, M.; Zhan, F.; Wood, A.J.J.; Stein, C.M. The effect of sildenafil on nitric oxide–mediated vasodilation in healthy men. Clin. Pharmacol. Ther. 2001, 70, 270–279. Available online: http://0-doi-wiley-com.brum.beds.ac.uk/10.1067/mcp.2001.117995 (accessed on 1 December 2021). [CrossRef]
- Sime, P.J.; Marr, R.A.; Gauldie, D.; Xing, Z.; Hewlett, B.R.; Graham, F.L.; Gauldie, J. Transfer of Tumor Necrosis Factor-α to Rat Lung Induces Severe Pulmonary Inflammation and Patchy Interstitial Fibrogenesis with Induction of Transforming Growth Factor-β1 and Myofibroblasts. Am. J. Pathol. 1998, 153, 825–832. [Google Scholar] [CrossRef]
- Benn, T.M.; Westerhoff, P. Nanoparticle Silver Released into Water from Commercially Available Sock Fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Air Pollution, the Automobile, and Public Health; Watson, A.Y.; Bates, R.R.; Kennedy, D. (Eds.) National Academies Press: Washington, DC, USA, 1988; Available online: http://www.nap.edu/catalog/1033 (accessed on 3 December 2021).
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midwood, K.S.; Piccinini, A.M. DAMPening Inflammation by Modulating TLR Signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar]
- Rosin, D.L.; Okusa, M.D. Dangers Within: DAMP Responses to Damage and Cell Death in Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 416–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, J.; Assenmacher, M.; Radbruch, A. Regulation of T helper cell cytokine expression: Functional dichotomy of antigen-presenting cells. Eur. J. Immunol. 1993, 23, 191–199. [Google Scholar] [CrossRef]
- Ghasemian, E.; Vatanara, A.; Rouini, M.R.; Najafabadi, A.R.; Gilani, K.; Lavasani, H.; Mohajel, N. Inhaled sildenafil nanocomposites: Lung accumulation and pulmonary pharmacokinetics. Pharm. Dev. Technol. 2015, 21, 961–971. Available online: https://0-www-tandfonline-com.brum.beds.ac.uk/doi/full/10.3109/10837450.2015.1086369 (accessed on 8 December 2021). [CrossRef]
- Otroj, M.; Taymouri, S.; Varshosaz, J.; Mirian, M. Preparation and characterization of dry powder containing sunitinib loaded PHBV nanoparticles for enhanced pulmonary delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101570. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S1773224719319434 (accessed on 9 December 2021). [CrossRef]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Muth, A.; Gupta, V. Sorafenib Loaded Inhalable Polymeric Nanocarriers against Non-Small Cell Lung Cancer. Pharm. Res. 2020, 37, 67. Available online: http://0-link-springer-com.brum.beds.ac.uk/10.1007/s11095-020-02790-3 (accessed on 10 December 2021). [CrossRef]
- Hakim, A.; Barnes, P.; Adcock, I.; Usmani, O. Abstracts from The Aerosol Society Drug Delivery to the Lungs 25Edinburgh International Conference Centre Edinburgh, Scotland, UK, 10–12 December 2014. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, A-1–A-25. Available online: http://www.liebertpub.com/doi/10.1089/jamp.2015.ab02.abstracts (accessed on 11 December 2021).
- Trotta, V.; Lee, W.H.; Loo, J.C.Y.; Young, P.; Traini, D.; Scalia, S. Co-spray dried resveratrol and budesonide inhalation formulation for reducing inflammation and oxidative stress in rat alveolar macrophages. Eur. J. Pharm. Sci. 2016, 86, 20–28. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0928098716300483 (accessed on 12 December 2021). [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Ma, X.; Su, Y.; Koleng, J.J.; Dolocan, A.; Williams, R.O. III. Using thin film freezing to minimize excipients in inhalable tacrolimus dry powder formulations. Int. J. Pharm. 2020, 586, 119490. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0378517320304749 (accessed on 13 December 2021). [CrossRef] [PubMed]
- Lee, C.; Seo, J.; Hwang, H.S.; Thao, L.Q.; Lee, S.; Lee, E.S.; Choi, H.-G.; Youn, Y.S. Treatment of bleomycin-induced pulmonary fibrosis by inhaled tacrolimus-loaded chitosan-coated poly (lactic-co-glycolic acid) nanoparticles. Biomed. Pharmacother. 2016, 78, 226–233. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0753332215302389 (accessed on 14 December 2021). [CrossRef] [PubMed]
- Seo, J.; Lee, C.; Hwang, H.S.; Kim, B.; Thao, L.Q.; Lee, E.S.; Oh, K.T.; Lim, J.-L.; Choi, H.-G.; Youn, Y.S. Therapeutic advantage of inhaled tacrolimus-bound albumin nanoparticles in a bleomycin-induced pulmonary fibrosis mouse model. Pulm. Pharmacol. Ther. 2016, 36, 53–61. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S1094553916300013 (accessed on 14 December 2021). [CrossRef] [PubMed]
- Watts, A.B.; Peters, J.I.; Talbert, R.L.; O’Donnell, K.P.; Coalson, J.J.; Williams, R.O. Preclinical evaluation of tacrolimus colloidal dispersion for inhalation. Eur. J. Pharm. Biopharm. 2011, 77, 207–215. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0939641110003073 (accessed on 15 December 2021). [CrossRef]
- Watts, A.B.; Cline, A.M.; Saad, A.R.; Johnson, S.B.; Peters, J.I.; Williams, R.O. Characterization and pharmacokinetic analysis of tacrolimus dispersion for nebulization in a lung transplanted rodent model. Int. J. Pharm. 2010, 384, 46–52. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0378517309007017 (accessed on 15 December 2021). [CrossRef]
- Lin, L.; Quan, G.; Peng, T.; Huang, Z.; Singh, V.; Lu, M.; Wu, C. Development of fine solid-crystal suspension with enhanced solubility, stability, and aerosolization performance for dry powder inhalation. Int. J. Pharm. 2017, 533, 84–92. [Google Scholar] [CrossRef]
- Rossi, I.; Sonvico, F.; McConville, J.T.; Rossi, F.; Fröhlich, E.; Zellnitz, S.; Rossi, A.; Del Favero, E.; Bettini, R.; Buttini, F. Nebulized coenzyme Q 10 nanosuspensions: A versatile approach for pulmonary antioxidant therapy. Eur. J. Pharm. Sci. 2018, 113, 159–170. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0928098717305791 (accessed on 16 December 2021). [CrossRef]
- Ohmori, Y.; Onoue, S.; Endo, K.; Matsumoto, A.; Uchida, S.; Yamada, S. Development of dry powder inhalation system of novel vasoactive intestinal peptide (VIP) analogue for pulmonary administration. Life Sci. 2006, 79, 138–143. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S002432050600004X (accessed on 17 December 2021). [CrossRef]
- Gupta, N.; Al-Saikhan, F.I.; Patel, B.; Rashid, J.; Ahsan, F. Fasudil and SOD packaged in peptide-studded-liposomes: Properties, pharmacokinetics and ex-vivo targeting to isolated perfused rat lungs. Int. J. Pharm. 2015, 488, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Wilson, J., Hunt, T., Eds.; Taylor & Francis Group: Abingdon, UK, 2017; Available online: https://www.taylorfrancis.com/books/9781317563754 (accessed on 17 December 2021).
- Boraschi, D.; Duschl, A. Nanoparticles and the Immune System: Safety and Effects; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Pallardy, M.J.; Turbica, I.; Biola-Vidamment, A. Why the Immune System Should Be Concerned by Nanomaterials? Front. Immunol. 2017, 8, 544. [Google Scholar] [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, T.; Nakamura, K.; Akagi, S.; Kusano, K.F.; Matsubara, H.; Fujio, H.; Ogawa, A.; Miura, A.; Miura, D.; Oto, T.; et al. Inhibitory Effects of Simvastatin on Platelet-derived Growth Factor Signaling in Pulmonary Artery Smooth Muscle Cells From Patients With Idiopathic Pulmonary Arterial Hypertension. J. Cardiovasc. Pharmacol. 2010, 55, 39–48. Available online: https://journals.lww.com/00005344-201001000-00007 (accessed on 17 December 2021). [CrossRef] [PubMed] [Green Version]
- Feng, R.; Zhang, Z.; Li, Z.; Huang, G. Preparation and in vitro evaluation of etoposide-loaded PLGA microspheres for pulmonary drug delivery. Drug Deliv. 2013, 21, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byron, P.R. Determinants of drug and polypeptide bioavailability from aerosols delivered to the lung. Adv. Drug Deliv. Rev. 1990, 5, 107–132. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/0169409X9090010P (accessed on 18 December 2021). [CrossRef]
- Davis, S.S. Delivery of peptide and non-peptide drugs through the respiratory tract. Pharm. Sci. Technol. Today 1999, 2, 450–456. [Google Scholar] [CrossRef]
- Scheuch, G.; Kohlhaeufl, M.J.; Brand, P.; Siekmeier, R. Clinical perspectives on pulmonary systemic and macromolecular delivery. Adv. Drug Deliv. Rev. 2006, 58, 996–1008. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0169409X0600144X (accessed on 18 December 2021). [CrossRef]
- Kojima, R.; Yoshida, T.; Tasaki, H.; Umejima, H.; Maeda, M.; Higashi, Y.; Watanabe, S.; Oku, N. Release mechanisms of tacrolimus-loaded PLGA and PLA microspheres and immunosuppressive effects of the microspheres in a rat heart transplantation model. Int. J. Pharm. 2015, 492, 20–27. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0378517315300259 (accessed on 19 December 2021). [CrossRef]
- Guichard, M.-J.; Leal, T.; Vanbever, R. PEGylation, an approach for improving the pulmonary delivery of biopharmaceuticals. Curr. Opin. Colloid Interface Sci. 2017, 31, 43–50. [Google Scholar] [CrossRef]
- Fröhlich, E.; Salar-Behzadi, S. Oral inhalation for delivery of proteins and peptides to the lungs. Eur. J. Pharm. Biopharm. 2021, 163, 198–211. [Google Scholar] [CrossRef]
- Bodier-Montagutelli, E.; Mayor, A.; Vecellio, L.; Respaud, R.; Heuzé-Vourc’H, N. Designing inhaled protein therapeutics for topical lung delivery: What are the next steps? Expert Opin. Drug Deliv. 2018, 15, 729–736. [Google Scholar] [CrossRef]
- Matthews, A.A.; Ee, P.L.R.; Ge, R. Developing inhaled protein therapeutics for lung diseases. Mol. Biomed. 2020, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Bukar, J.; Nagarajan, S. Inhaled insulin. Adv. Drug Deliv. Rev. 1999, 35, 235–247. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0169409X9800074X (accessed on 19 December 2021). [CrossRef]
- Sánchez, A.; Villamayor, B.; Guo, Y.; McIver, J.; Alonso, M.J. Formulation strategies for the stabilization of tetanus toxoid in poly(lactide-co-glycolide) microspheres. Int. J. Pharm. 1999, 185, 255–266. [Google Scholar] [CrossRef]
- Thanoo, B.C.; Sunny, M.C.; Jayakrishnan, A. Cross-linked Chitosan Microspheres: Preparation and Evaluation as a Matrix for the Controlled Release of Pharmaceuticals. J. Pharm. Pharmacol. 1992, 44, 283–286. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kabe, J.; Noda, M.; Kobayashi, N.; Miura, K. Physiologic and pathologic respiratory changes in delayed type hypersensitivity reaction in guinea pigs. Am. Rev. Respir. Dis. 1971, 103, 509–515. [Google Scholar]
- Bukowski, R.M.; Tendler, C.; Cutler, D.; Rose, E.; Laughlin, M.M.; Statkevich, P. Treating cancer with PEG Intron. Cancer 2002, 95, 389–396. [Google Scholar] [CrossRef]
- Rich, S.; McLaughlin, V.V. The effects of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertension. J. Am. Coll. Cardiol. 1999, 34, 1184–1187. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, D.; Gaine, S.P. Combination Therapy and New Types of Agents for Pulmonary Arterial Hypertension. Clin. Chest Med. 2007, 28, 169–185. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0272523106001262 (accessed on 20 December 2021). [CrossRef]
- Skoro-Sajer, N. Optimal Use of Treprostinil in Pulmonary Arterial Hypertension. Drugs 2012, 72, 2351–2363. Available online: http://0-link-springer-com.brum.beds.ac.uk/10.2165/11638260-000000000-00000 (accessed on 21 December 2021). [CrossRef]
- Sosnowski, T.R. Inhaled aerosols: Their role in COVID-19 transmission, including biophysical interactions in the lungs. Curr. Opin. Colloid Interface Sci. 2021, 54, 101451. [Google Scholar] [CrossRef]
- Hu, X.; Yang, F.-F.; Quan, L.-H.; Liu, C.-Y.; Liu, X.-M.; Ehrhardt, C.; Liao, Y.-H. Pulmonary delivered polymeric micelles – Pharmacokinetic evaluation and biodistribution studies. Eur. J. Pharm. Biopharm. 2014, 88, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.; Yang, R.; Chu, K.; Yu, A.; Adi, S.; Chan, H. Numerical study of the effects of particle size and polydispersity on the agglomerate dispersion in a cyclonic flow. Chem. Eng. J. 2010, 164, 432–441. [Google Scholar] [CrossRef]
- Tong, Z.B.; Adi, S.; Yang, R.Y.; Chan, H.-K.; Yu, A. Numerical investigation of the de-agglomeration mechanisms of fine powders on mechani-cal impaction. J. Aerosol Sci. 2011, 42, 811–819. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Moskal, A.; Gradoń, L. Mechanims of Aerosol Particle Deposition in the Oro-Pharynx Under Non-Steady Airflow. Ann. Occup. Hyg. 2007, 51, 19–25. [Google Scholar] [PubMed]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O., III. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0378517311000147 (accessed on 21 December 2021). [CrossRef]
- Sosnowski, T.R.; Odziomek, M. Particle Size Dynamics: Toward a Better Understanding of Electronic Cigarette Aerosol Interactions with the Respiratory System. Front. Physiol. 2018, 9, 853. Available online: https://www.frontiersin.org/article/10.3389/fphys.2018.00853/full (accessed on 22 December 2021). [CrossRef]
- Bake, B.; Larsson, P.; Ljungkvist, G.; Ljungström, E.; Olin, A.-C. Exhaled particles and small airways. Respir. Res. 2019, 20, 8. Available online: https://0-respiratory--research-biomedcentral-com.brum.beds.ac.uk/articles/10.1186/s12931-019-0970-9 (accessed on 22 December 2021). [CrossRef] [Green Version]
- Hinds, W.C. Aerosol Technology: Properties, Behaviour, and Measurement of Airborne Particles; Wiley: Hoboken, NJ, USA, 1982. [Google Scholar]
- Rostami, A.A. Computational Modeling of Aerosol Deposition in Respiratory Tract: A Review. Inhal. Toxicol. 2009, 21, 262–290. [Google Scholar] [CrossRef]
- Malcolmson, R.J.; Embleton, J.K. Dry powder formulations for pulmonary delivery. Pharm. Sci. Technol. Today 1998, 1, 394–398. [Google Scholar] [CrossRef]
- Papineni, R.S.; Rosenthal, F.S. The Size Distribution of Droplets in the Exhaled Breath of Healthy Human Subjects. J. Aerosol Med. 1997, 10, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Gralton, J.; Tovey, E.; McLaws, M.-L.; Rawlinson, W.D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Hanes, J.; Caponetti, G.; Hrkach, J.; Ben-Jebria, A.; Eskew, M.L.; Mintzes, J.; Deaver, D.; Lotan, N.; Langer, R. Large Porous Particles for Pulmonary Drug Delivery. Science 1997, 276, 1868–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, A.; Majumder, Q.H.; Ahsan, F. Inhalable large porous microspheres of low molecular weight heparin: In vitro and in vivo evaluation. J. Control. Release 2008, 128, 224–232. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0168365908001545 (accessed on 22 December 2021). [CrossRef] [Green Version]
- Haughney, J.; Price, D.; Barnes, N.C.; Virchow, J.C.; Roche, N.; Chrystyn, H. Choosing inhaler devices for people with asthma: Current knowledge and outstanding research needs. Respir. Med. 2010, 104, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Van Holsbeke, C.; Marshall, J.; De Backer, J.; Vos, W. Median mass aerodynamic diameter (MMAD) and fine particle fraction (FPF): Influence on lung deposition? Eur. Respir. J. 2014, 44, P912. [Google Scholar]
- Zeman, K.L.; Wu, J.; Bennett, W.D. Targeting Aerosolized Drugs to the Conducting Airways Using Very Large Particles and Extremely Slow Inhalations. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 363–369. Available online: http://www.liebertpub.com/doi/10.1089/jamp.2008.071 (accessed on 22 December 2021). [CrossRef]
- Pilcer, G.; Wauthoz, N.; Amighi, K. Lactose characteristics and the generation of the aerosol. Adv. Drug Deliv. Rev. 2012, 64, 233–256. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.-T.; Yi, E.-J.; Hwang, K.-M.; Cho, C.-H.; Park, C.-W.; Kim, J.-Y.; Rhee, Y.-S.; Park, E.-S. Formulation and evaluation of carrier-free dry powder inhaler containing sildenafil. Drug Deliv. Transl. Res. 2018, 9, 319–333. Available online: http://0-link-springer-com.brum.beds.ac.uk/10.1007/s13346-018-0586-5 (accessed on 2 January 2021). [CrossRef]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef]
- Snell, N.J.C.; Ganderton, D. Assessing lung deposition of inhaled medications: Consensus statement from a workshop of the British Association for Lung Research, held at the Institute of Biology, London, U.K. on 17 April 1998. Respir. Med. 1999, 93, 123–133. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0954611199903025 (accessed on 2 January 2021). [CrossRef] [Green Version]
- Baginski, L.; Gobbo, O.; Tewes, F.; Salomon, J.J.; Healy, A.M.; Bakowsky, U.; Ehrhardt, C. In Vitro and In Vivo Characterisation of PEG-Lipid-Based Micellar Complexes of Salmon Calcitonin for Pulmonary Delivery. Pharm. Res. 2012, 29, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, M.; Italia, J.; Mullen, A.; Kumar, M.R.; Candlish, A.; Williams, R.; Shaw, C.; Al Gawhari, F.; Coombs, G.; Wiese, M.; et al. The efficacy of aerosol treatment with non-ionic surfactant vesicles containing amphotericin B in rodent models of leishmaniasis and pulmonary aspergillosis infection. J. Control. Release 2012, 160, 685–691. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0168365912002453 (accessed on 3 January 2021). [CrossRef] [PubMed]
- Zaru, M.; Mourtas, S.; Klepetsanis, P.; Fadda, A.M.; Antimisiaris, S.G. Liposomes for drug delivery to the lungs by nebulization. Eur. J. Pharm. Biopharm. 2007, 67, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Nikander, K.; Berg, E.; Smaldone, G.C. Jet Nebulizers versus Pressurized Metered Dose Inhalers with Valved Holding Cham-bers: Effects of The Facemask on Aerosol Delivery. J. Aerosol Med. 2007, 20 (Suppl. 1), S46–S58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhand, R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir. Care 2002, 47, 1406–1416. [Google Scholar]
- Vallorz, E.; Sheth, P.; Myrdal, P. Pressurized Metered Dose Inhaler Technology: Manufacturing. AAPS PharmSciTech 2019, 20, 177. [Google Scholar] [CrossRef]
- Noriega-Fernandes, B.; Malmlöf, M.; Nowenwik, M.; Gerde, P.; Corvo, M.L.; Costa, E. Dry powder inhaler formulation comparison: Study of the role of particle deposition pattern and dissolution. Int. J. Pharm. 2021, 607, 121025. [Google Scholar] [CrossRef]
- Memisoglu-Bilensoy, E.; Vural, I.; Bochot, A.; Renoir, J.M.; Duchene, D.; Hıncal, A.A. Tamoxifen citrate loaded amphiphilic β-cyclodextrin nanoparticles: In vitro characterization and cytotoxicity. J. Control. Release 2005, 104, 489–496. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0168365905001203 (accessed on 10 January 2021). [CrossRef]
- Ewert, R.; Gläser, S.; Bollmann, T.; Schäper, C. Inhaled iloprost for therapy in pulmonary arterial hypertension. Expert Rev. Respir. Med. 2011, 5, 145–152. [Google Scholar] [CrossRef]
- Channick, R.; Voswinckel; Rubin, L. Inhaled treprostinil: A therapeutic review. Drug Des. Dev. Ther. 2012, 2012, 19. Available online: http://www.dovepress.com/inhaled-treprostinil-a-therapeutic-review-peer-reviewed-article-DDDT (accessed on 12 January 2021). [CrossRef] [PubMed] [Green Version]
- Agnoletti, M.; Bohr, A.; Thanki, K.; Wan, F.; Zeng, X.; Boetker, J.; Yang, M.; Foged, C. Inhalable siRNA-loaded nano-embedded microparticles engineered using microfluidics and spray drying. Eur. J. Pharm. Biopharm. 2017, 120, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors affecting microencapsulation of drugs in liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.P.; Dupont, L.; Konstan, M.W.; Billings, J.; Fustik, S.; Goss, C.H.; Lymp, J.; Minic, P.; Quittner, A.L.; Rubenstein, R.C.; et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 2013, 68, 818–825. [Google Scholar] [CrossRef] [Green Version]
- Verschraegen, C.F.; Gilbert, B.E.; Loyer, E.; Huaringa, A.; Walsh, G.; Newman, R.A.; Knight, V. Clinical Evaluation of the Delivery and Safety of Aerosolized Liposomal 9-Nitro-20(S)-Camptothecin in Patients with Advanced Pulmonary Malignancies. Clin. Cancer Res. 2004, 10, 2319–2326. [Google Scholar] [CrossRef] [Green Version]
- Rashid, J.; Nahar, K.; Raut, S.; Keshavarz, A.; Ahsan, F. Fasudil and DETA NONOate, Loaded in a Peptide-Modified Liposomal Carrier, Slow PAH Progression upon Pulmonary Delivery. Mol. Pharm. 2018, 15, 1755–1765. [Google Scholar] [CrossRef]
- Keshavarz, A.; Kadry, H.; Alobaida, A.; Ahsan, F. Newer approaches and novel drugs for inhalational therapy for pulmonary arterial hypertension. Expert Opin Drug Deliv. 2020, 17, 439–461. [Google Scholar] [CrossRef]
- Peacock, A.J.; Murphy, N.F.; McMurray, J.J.V.; Caballero, L.; Stewart, S. An epidemiological study of pulmonary arterial hypertension. Eur. Respir. J. 2007, 30, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; Montani, D.; Celermajer, D.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Toba, M.; Alzoubi, A.; O’Neill, K.D.; Gairhe, S.; Matsumoto, Y.; Oshima, K.; Abe, K.; Oka, M.; McMurtry, I.F. Temporal hemodynamic and histological progression in Sugen5416/hypoxia/normoxia-exposed pulmonary arterial hypertensive rats. Am. J. Physiol. Circ. Physiol. 2014, 306, H243–H250. [Google Scholar] [CrossRef] [Green Version]
- Tsifansky, M.D.; Yeo, Y.; Evgenov, O.V.; Bellas, E.; Benjamin, J.; Kohane, D.S. Microparticles for Inhalational Delivery of Antipseudomonal Antibiotics. AAPS J. 2008, 10, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, X.; Shi, X.; Li, Z.; Sun, Y. Effects of magnetic dihydroartemisinin nano-liposome in inhibiting the proliferation of head and neck squamous cell carcinomas. Phytomedicine 2018, 56, 215–228. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0944711318305646 (accessed on 20 January 2021). [CrossRef] [PubMed]
- Varrica, C.; Carvalheiro, M.; Faria-Silva, C.; Eleutério, C.; Sandri, G.; Simões, S. Topical Allopurinol-Loaded Nanostructured Lipid Carriers: A Novel Approach for Wound Healing Management. Bioengineering 2021, 8, 192. Available online: https://0-www-mdpi-com.brum.beds.ac.uk/2306-5354/8/12/192 (accessed on 23 January 2021). [CrossRef] [PubMed]
- Chen, S.; Zhang, Y.; Qing, J.; Han, Y.; McClements, D.J.; Gao, Y. Core-shell nanoparticles for co-encapsulation of coenzyme Q10 and piperine: Surface engineering of hydrogel shell around protein core. Food Hydrocoll. 2020, 103, 105651. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0268005X19321940 (accessed on 25 January 2021). [CrossRef]
- Hu, X.; Han, R.; Quan, L.-H.; Liu, C.-Y.; Liao, Y.-H. Stabilization and sustained release of zeylenone, a soft cytotoxic drug, within polymeric micelles for local antitumor drug delivery. Int. J. Pharm. 2013, 450, 331–337. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0378517313002998 (accessed on 29 January 2021). [CrossRef]
- Geiser, M.; Kreyling, W.G. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.O.; Kabanov, A.; Bronich, T.K. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J. Control. Release 2009, 138, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic® block copolymers. J. Control. Release 2004, 94, 411–422. [Google Scholar] [CrossRef]
- Gaber, N.N.; Darwis, Y.; Peh, K.-K.; Tan, Y.T.-F. Characterization of Polymeric Micelles for Pulmonary Delivery of Beclomethasone Dipropionate. J. Nanosci. Nanotechnol. 2006, 6, 3095–3101. Available online: https://0-www-ingentaconnect-com.brum.beds.ac.uk/content/10.1166/jnn.2006.426 (accessed on 2 February 2021). [CrossRef]
- Righeschi, C.; Coronnello, M.; Mastrantoni, A.; Isacchi, B.; Bergonzi, M.C.; Mini, E.; Bilia, A.R. Strategy to provide a useful solution to effective delivery of dihydroartemisinin: Development, characterization and in vitro studies of liposomal formulations. Colloids Surf. B Biointerfaces 2014, 116, 121–127. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0927776513007674 (accessed on 12 February 2021). [CrossRef]
- Lukyanov, A.N.; Gao, Z.; Torchilin, V.P. Micelles from polyethylene glycol/phosphatidylethanolamine conjugates for tumor drug delivery. J. Control. Release 2003, 91, 97–102. [Google Scholar] [CrossRef]
- Gill, K.K.; Nazzal, S.; Kaddoumi, A. Paclitaxel loaded PEG5000–DSPE micelles as pulmonary delivery platform: Formulation characterization, tissue distribution, plasma pharmacokinetics, and toxicological evaluation. Eur. J. Pharm. Biopharm. 2011, 79, 276–284. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S0939641111001597 (accessed on 20 February 2021). [CrossRef] [PubMed]
- Zamani, M.; Aghajanzadeh, M.; Rostamizadeh, K.; Manjili, H.K.; Fridoni, M.; Danafar, H. In vivo study of poly (ethylene glycol)-poly (caprolactone)-modified folic acid nanocarriers as a pH responsive system for tumor-targeted co-delivery of tamoxifen and quercetin. J. Drug Deliv. Sci. Technol. 2019, 54, 101283. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/S1773224719309293 (accessed on 21 February 2021). [CrossRef]
- Yao, D.G.; Sun, K.X.; Mu, H.J.; Zhou, F.M.; Chen, H.H.; Liu, L.J.; Liang, N. Preparation of cyclosporine a loaded mPEG-PLGA copolymer micelles and study its phar-macokinetics in rats. Yaoxue Xuebao 2009, 44, 1410–1415. [Google Scholar]
- Patel, B.; Gupta, V.; Ahsan, F. PEG–PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J. Control. Release 2012, 162, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Chokamonsirikun, A.; Phattaravorakarn, V.; Tiyaboonchai, W. Polymeric micelles for pulmonary drug delivery: A comprehensive review. J. Mater. Sci. 2020, 56, 2016–2036. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Möller, W. Ultrafine Particle–Lung Interactions: Does Size Matter? J. Aerosol Med. 2006, 19, 74–83. [Google Scholar] [CrossRef]
- Anderson, N.S.; Silva, R.M.; Lee, D.; Edwards, P.C.; Sharmah, A.; Guo, T.; Pinkerton, K.E.; Van Winkle, L.S. Persistence of silver nanoparticles in the rat lung: Influence of dose, size, and chemical composition. Nanotoxicology 2014, 9, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Huang, Y.; Wang, W.; Fu, F.; Dang, S.; Li, C.; Ma, C.; Zhang, X.; Zhao, Z.; Pan, X.; et al. Relationship between particle size and lung retention time of intact solid lipid nanoparticle suspensions after pulmonary delivery. J. Control. Release 2020, 325, 206–222. [Google Scholar] [CrossRef]




| MOLECULE | Formulation | Aerodynamic Behavior | Method of Preparation | Characteristics | References | ||
|---|---|---|---|---|---|---|---|
| Zeta mV | Size (µm) | EE/LE (%) | |||||
| SILDENAFIL CITRATE | Sildenafil Citrate -loaded PLGA nanoparticles | FPF = 31.8–60.2% | Double emulsion solvent evaporation | - | 4.2–18.1 | [58] | |
| SUNITINIB | Sunitinib loaded poly (3-hydroxybutyrate-co-3-hydroxyvalerate acid) nanoparticles (nps) | MMAD = 3.38 ± 0.06 μm, FPF = 61.18 ± 0.04% | Solvent-emulsification evaporation | −1.37 ± 0.24 mv | 167.80 ± 0.30 nm | 93.25 ± 0.03% | [59] |
| SORAFENIB | Sorafenib-loaded cationically modified polymeric nanoparticles | Mmad = 4 μm FPF > 80% | Solvent-evaporation | −21.5 ± 0.7 mv and −32.9 ± 2.9 mv | 196.1 ± 1.1 nm (PDI 0.04 ± 0.02) and 191.4 ± 10.1 nm | 38.2 ± 0.6% | [60] |
| RESVERATROL (RES) | Novel spray dried inhalation powder | MMAD = 3.86 ± 1.04 μm FPF = 39.89 ± 1.06% | Spray drying | −1.46 ± 1.47 | <5 μm | 29.1 ± 2.0 | [61] |
| RESVERATROL (RES) | Co-spray dried (Co-SD) formulations of and budesonide and resveratrol | FPF = 42.5 ± 1.7% | Spray drying | - | 1 to 5 μm | - | [62] |
| TACROLIMUS | Dry powder inhaler | Good Mobility of aerosol | Thin film freezing | - | - | - | [63] |
| Chitosan Tacrolimus PLGA-nps | Aerosol particles with good mobility | Oil/water emulsification diffusion method | +13.6 | 441 | 37.7 | [64] | |
| Nanoparticles | Aerosol particle at inhalable range | Modification of albumin-bound technology | −34.5 ± 0.3 mv | 182.1 ± 28.5 | 85.3 ± 4.7 | [65] | |
| Colloidal dispersion, powder for reconstitution inhalation | 46.1% of the emitted dose was in the respirable range | Ultra-rapid freezing (URF) process | - | 200 to 400 | - | [66] | |
| Tacrolimus powder for reconstitution in deionized water | MMAD = 4.06 µm GSD = 2.7 µm. FPF = 46.1% | Thin film freezing (TFF) | - | 239.2 | - | [67] | |
| Inhalable albumin nanoparticles with bound Tacrolimus | Aerosolisation maintained for ~0.12 s after actuation, 0.04 sec intervals | High-pressure homogenization technique | −34.5 ± 0.3 mv | 182.1 ± 28.5 | 85.3% | [59] | |
| COENZYME Q10 | Microparticles | Aerosol particle at inhalable range | High-pressure homogenizer | - | - | - | [68] |
| Nanosuspensions | Good aerodynamic Size | High-pressure homogenizer (HPH) | -20 | 100 | [69] | ||
| VIP | Dry powder formulation | Stage 3 (3.3–4.7 μm) aerodynamic size less than 10 μm, | Milled with an A-O JET MILL | - | 4.5 μm | - | [70] |
| CARSKNKDC (CAR) * | Liposomes | That energy produced by the microsprayer did not affect liposomal Integrity | Thin-film formation, hydration, and extrusion method | 152.7 ± 2.38 nm | 54.91 ± 1.66 (superoxide Dismutase) 39.22 ± 3.41 (Fasudil) | [71] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnihotri, V.; Agrawal, Y.; Goyal, S.; Sharma, C.; Ojha, S. An Update on Advancements and Challenges in Inhalational Drug Delivery for Pulmonary Arterial Hypertension. Molecules 2022, 27, 3490. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113490
Agnihotri V, Agrawal Y, Goyal S, Sharma C, Ojha S. An Update on Advancements and Challenges in Inhalational Drug Delivery for Pulmonary Arterial Hypertension. Molecules. 2022; 27(11):3490. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113490
Chicago/Turabian StyleAgnihotri, Vinit, Yogeeta Agrawal, Sameer Goyal, Charu Sharma, and Shreesh Ojha. 2022. "An Update on Advancements and Challenges in Inhalational Drug Delivery for Pulmonary Arterial Hypertension" Molecules 27, no. 11: 3490. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27113490