The Possibility of Using Waste Phosphates from the Production of Polyols for Fertilizing Purposes
Abstract
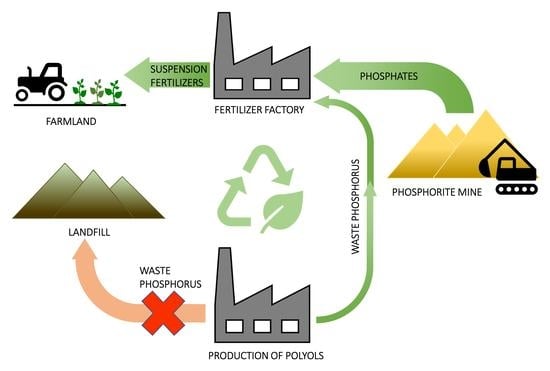
:1. Introduction
2. Results and Discussion
2.1. Organic Carbon Content Analysis Results
2.2. The Results of the Chemical Analysis of the Waste
2.3. The Results of the Sediment Composition Analysis in the Waste
3. Materials and Methods
3.1. Methods for the Analysis of Organic Carbon Content
3.2. Methods of Chemical Analysis of Waste
3.3. Methods of Sludge-in-Waste Analysis
- 0.04 rad soller gap;
- mask: 5 mm;
- slit for beam divergence adjustment: 1/2⁰;
- anti-scatter gap: 1⁰.
- 0.04 rad soller gap;
- anti-scatter gap: 8 mm PIXel 3D;
- elliptical mirror.
- initial angle [⁰2θ]: 5;
- end angle [⁰2θ]: 75;
- scan step [⁰2θ]: 0.0394;
- exposure time [s]: 68.6;
- sample rotation: yes, disc rotation with a period of 4 s.
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ciećko, Z.; Najmowicz, T.; Wyszkowski, M.; Markiewicz, K. Oddziaływanie zanieczyszczenia gleby arsenem na zawartość fosforu w roślinach. Pr. Nauk. Uniw. Ekon. We Wrocławiu 2008, 4, 60–70. [Google Scholar]
- Łuczkowska, D.; Kużdżał, E.; Cichy, B. Study on the reactivity of phosphorites. Przem. Chem. 2016, 95, 1538–1541. [Google Scholar] [CrossRef]
- Smol, M. The importance of sustainable phosphorus management in the circular economy (CE) model: The Polish case study. J. Mater. Cycles Waste Manag. 2019, 21, 227–238. [Google Scholar] [CrossRef]
- EC. Komunikat Komisji do Parlamentu Europejskiego, Rady, Europejskiego Komitetu Ekonomiczno-Społecznego i Komitetu regionów. Komunikat Konsultacyjny w Sprawie Zrównoważonego Stosowania Fosforu. 2013. Available online: https://eur-lex.europa.eu/legal-content/PL/ALL/?uri=CELEX%3A52013DC0517 (accessed on 14 July 2022).
- Smol, M.; Kulczycka, J.; Czaplicka-Kotas, A.; Włóka, D. Zarządzanie i monitorowanie gospodarki odpadami komunalnymi w Polsce w kontekście realizacji gospodarki o obiegu zamkniętym (GOZ). Zesz. Nauk. Inst. Gospod. Surowcami Miner. Pol. Akad. Nauk 2019, 108, 165–184. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crops Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. The Possibility of Organo-Mineral Fertilizer Production from Sewage Sludge. Waste Biomass Valorization 2017, 8, 1781–1791. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Erisman, J.W.; Abrol, Y.P.; Bekunda, M.; Datta, A.; Davidson, E.; De Vries, W.; Oenema, O.; et al. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution; Centre for Ecology and Hydrology (CEH): Edinburgh, UK, 2013. [Google Scholar]
- Rolewicz, M.; Rusek, P.; Mikos-Szymańska, M.; Cichy, B.; Dawidowicz, M. Obtaining of Suspension Fertilizers from Incinerated Sewage Sludge Ashes (ISSA) by a Method of Solubilization of Phosphorus Compounds by Bacillus megaterium Bacteria. Waste Biomass Valorization 2016, 7, 871–877. [Google Scholar] [CrossRef]
- Cichy, B.; Jaroszek, H.; Paszek, A.; Tarnowska, A. XV Konferencja Ochrona Środowiska Kadm w nawozach fosforowych; aspekty ekologiczne i ekonomiczne. Chemik 2014, 68, 837–839. [Google Scholar]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact to 2050. Glob. Food Sec. 2020, 26, 100426. [Google Scholar] [CrossRef]
- Golroudbary, S.R.; El Wali, M.; Kraslawski, A. Rationality of using phosphorus primary and secondary sources in circular economy: Game-theory-based analysis. Environ. Sci. Policy 2020, 106, 166–176. [Google Scholar] [CrossRef]
- Slootweg, J.C. Using waste as resource to realize a circular economy: Circular use of C, N and P. Curr. Opin. Green Sustain. Chem. 2020, 23, 61–66. [Google Scholar] [CrossRef]
- Seroka-Stolka, O.; Ociepa-Kubicka, A. Green logistics and circular economy. Transp. Res. Procedia 2019, 39, 471–479. [Google Scholar] [CrossRef]
- Kasprzyk, M.; Czerwionka, K.; Gajewska, M. Waste materials assessment for phosphorus adsorption toward sustainable application in circular economy. Resour. Conserv. Recycl. 2021, 168, 105335. [Google Scholar] [CrossRef]
- Martins, F.F.; Castro, H. Raw material depletion and scenario assessment in European Union—A circular economy approach. Energy Rep. 2020, 6, 417–422. [Google Scholar] [CrossRef]
- Smol, M.; Kulczycka, J. Możliwości Wykorzystania Odpadów Jako Źródła Surowców Krytycznych w Sektorze Nawozowym—Wdrażanie Założeń Gospodarki o Obiegu Zamkniętym (GOZ) na Przykładzie Fosforu. Mikrozanieczyszczenia W Ściekach Odpad. I Sr. 2018, 108, 331–348. Available online: https://www.researchgate.net/publication/334544880_Mozliwosci_wykorzystania_odpadow_jako_zrodla_surowcow_krytycznych_w_sektorze_nawozowym_-_wdrazanie_zalozen_gospodarki_o_obiegu_zamknietym_GOZ_na_przykladzie_fosforu_Possibilities_in_the_use_of_waste_a (accessed on 18 July 2022).
- Górecki, H.; Hoffoann, J. Nawozy zawiesinowe—Nowa generacja nawozów rolniczych i ogrodniczych. Przem. Chem. 1995, 3, 87–90. [Google Scholar]
- Mikła, D.; Hoffmann, K.; Hoffmann, J. Production of suspension fertilizers as a potential way of managing industrial waste. Pol. J. Chem. Technol. 2007, 9, 9–11. [Google Scholar] [CrossRef]
- Malinowski, P.; Olech, M.; Sas, J.; Wantuch, W.; Biskupski, A.; Urbańczyk, L.; Borowik, M.; Kotowicz, J. Production of compound mineral fertilizers as a method of utilization of waste products in chemical company Alwernia SA. Polish J. Chem. Technol. 2010, 12. [Google Scholar] [CrossRef]
- Malinowski, P.; Olech, M.; Biskupski, A. Ochrona Środowiska Źródłem Innowacji—Rozwiązania Wdrożone W Alwerni S.A. 2010, pp. 122–129. Available online: http://www.ptzp.org.pl/files/konferencje/kzz/artyk_pdf_2014/T1/t1_122.pdf (accessed on 18 July 2022).
- Rusek, P.; Biskupski, A.; Borowik, M.; Hoffmann, J. Rozwoj techologii wytwarzania nawozow zawiesinowych. Przem. Chem. 2009, 88, 1332–1335. [Google Scholar]
- Mikła, D. Zawiesinowy Nawóz Wieloskładnikowy. OPIS PATENTOWY PL 230018, 2 January 2014. Available online: https://chemfari.pl/wp-content/uploads/2018/10/Patent-nr-230018.pdf (accessed on 18 July 2022).
- Regulation EU. Regulation of the european parliament and of the council laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 2019, 114. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1009&from=EN (accessed on 18 July 2022).
- Bogusz, P.; Rusek, P.; Brodowska, M.S. Suspension fertilizers: How to reconcile sustainable fertilization and environmental protection. Agriculture 2021, 11, 1008. [Google Scholar] [CrossRef]





| Sample | TOC [ppm] | Average |
|---|---|---|
| 1 | 137 | 151 |
| 137 | ||
| 178 | ||
| 2 | 246 | 276 |
| 239 | ||
| 344 | ||
| 3 | 457 | 461 |
| 460 | ||
| 467 | ||
| 4 | 383 | 383 |
| 372 | ||
| 394 | ||
| 5 | 445 | 419 |
| 436 | ||
| 377 | ||
| 6 | 318 | 355 |
| 344 | ||
| 402 | ||
| Average | 341 |
| Sample | TOC [ppm] | Average |
|---|---|---|
| 1 | 555 | 574 |
| 628 | ||
| 572 | ||
| 2 | 592 | 581 |
| 616 | ||
| 557 | ||
| 3 | 555 | 585 |
| 628 | ||
| 572 | ||
| Average | 586 |
| Tested Feature | Unit | Result y ± U 1 | Requirements for Suspension Fertilizers According to EU Regulation No. 2019/1009 |
|---|---|---|---|
| Phosphorus soluble in mineral acids expressed as P2O5 | % | 23.46 ± 0.3 | min. 1.5% by mass of total phosphorus pentoxide (P2O5) |
| Potassium as K2O | % | 9.89 ± 0.16 | min. 1.5% by mass of total potassium oxide (K2O) |
| Sodium (Na) | % | 7.60 | min. 0.5%-max. 20% by mass of total sodium oxide (Na2O) |
| Copper (Cu) | mg/kg | less than 1.0 | 6002 |
| Iron (Fe) | mg/kg | 46.0 | - |
| Zinc (Zn) | mg/kg | less than 1.0 | 15002 |
| Cadmium (Cd) | mg/kg | less than 1.0 | 60 |
| Lead (Pb) | mg/kg | less than 8.0 | 120 |
| Nickel (Ni) | mg/kg | less than 1.0 | 100 |
| Aluminum (Al) | mg/kg | 74.7 | - |
| Arsen (As) | mg/kg | less than 4.0 | 40 |
| Mercury (Hg) | mg/kg | less than 0.002 | 1 |
| Chrome (VI) | mg/kg | less than 0.3 | 2 |
| Tested Feature | Test Method | Procedure |
|---|---|---|
| Phosphorus soluble in mineral acids expressed as P2O5 | weight method | PN-EN 15956:2011 PN-EN 15959:2011 |
| Potassium expressed as K2O | weight method | PN-EN 15477:2009 |
| Sodium (Na) Copper (Cu) Iron (Fe) Zinc (Zn) Cadmium (Cd) Lead (Pb) Nickel (Ni) Aluminum (Al) | inductively coupled plasma atomic emission spectrometry (ICP-OES) | PB 35 ed. III of 02/03/2020 PN-EN 16319 + A1: 2016-02 with the exception of point 8.2 |
| Arsen (As) | inductively coupled plasma atomic emission spectrometry (ICP-OES) | PB 35 ed. II of 02/03/2020 PN-EN 16317 + A1: 2017-04 with the exception of point 8.2 |
| Mercury (Hg) | atomic absorption spectrometry with the amalgamation technique | RMG annex 3, p. 4 * |
| Chrome (VI) | ion chromatography | PN-EN 16318+A1:2016-03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusz, P. The Possibility of Using Waste Phosphates from the Production of Polyols for Fertilizing Purposes. Molecules 2022, 27, 5632. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27175632
Bogusz P. The Possibility of Using Waste Phosphates from the Production of Polyols for Fertilizing Purposes. Molecules. 2022; 27(17):5632. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27175632
Chicago/Turabian StyleBogusz, Paulina. 2022. "The Possibility of Using Waste Phosphates from the Production of Polyols for Fertilizing Purposes" Molecules 27, no. 17: 5632. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27175632