Quantification of Porous Properties of Shear Crystallized Lipids
Abstract
:1. Introduction
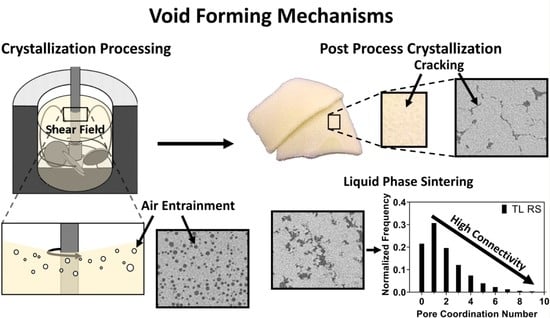
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Crystallization Process
3.3. Solid Fat Content
3.4. Thermal Properties
3.5. Crystal Morphology
3.6. Polymorphic Behavior
3.7. Pore Network Measurements
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Acevedo, N.; Maleky, F.; Co, E.; Peyronel, F.; Mazzanti, G.; Quinn, B.; Pink, D. Structure and functionality of edible fats. Soft. Matter. 2012, 8, 1275–1300. [Google Scholar] [CrossRef]
- Martini, S.; Herrera, M.L.; Hartel, R.W. Effect of Cooling Rate on Nucleation Behavior of Milk Fat−Sunflower Oil Blends. J. Agric. Food Chem. 2001, 49, 3223–3229. [Google Scholar] [CrossRef]
- Mazzanti, G.; Marangoni, A.G.; Idziak, S.H.J. Modeling phase transitions during the crystallization of a multicomponent fat under shear. Phys. Rev. E 2005, 71, 041607. [Google Scholar] [CrossRef]
- Mudge, E.M.; Mazzanti, G. Rheo-NMR Measurements of Cocoa Butter Crystallized Under Shear Flow. Cryst. Growth Des. 2009, 9, 3111–3118. [Google Scholar] [CrossRef]
- Maleky, F.; Marangoni, A. Thermal and Mechanical Properties of Cocoa Butter Crystallized under an External Laminar Shear Field. Cryst. Growth Des. 2011, 11, 2429–2437. [Google Scholar] [CrossRef]
- Maleky, F.; Smith, A.K.; Marangoni, A. Laminar Shear Effects on Crystalline Alignments and Nanostructure of a Triacylglycerol Crystal Network. Cryst. Growth Des. 2011, 11, 2335–2345. [Google Scholar] [CrossRef]
- Maleky, F.; Acevedo, N.C.; Marangoni, A.G. Cooling rate and dilution affect the nanostructure and microstructure differently in model fats. Eur. J. Lipid Sci. Technol. 2012, 114, 748–759. [Google Scholar] [CrossRef]
- Campos, R.; Marangoni, A.G. Crystallization Dynamics of Shear Worked Cocoa Butter. Cryst. Growth Des. 2014, 14, 1199–1210. [Google Scholar] [CrossRef]
- Zulkurnain, M.; Balasubramaniam, V.M.; Maleky, F. Thermal Effects on Lipids Crystallization Kinetics under High Pressure. Cryst. Growth Des. 2017, 17, 4835–4843. [Google Scholar] [CrossRef]
- Bourlieu, C.; Guillard, V.; Powell, H.; Vallès-Pàmies, B.; Guilbert, S.; Gontard, N. Performance of lipid-based moisture barriers in food products with intermediate water activity. Eur. J. Lipid Sci. Technol. 2006, 108, 1007–1020. [Google Scholar] [CrossRef]
- Maleky, F.; Marangoni, A. Nanoscale effects on oil migration through triacylglycerol polycrystalline colloidal networks. Soft. Matter. 2011, 7, 6012. [Google Scholar] [CrossRef]
- Dahlenborg, H.; Millqvist-Fureby, A.; Bergenståhl, B. Effect of particle size in chocolate shell on oil migration and fat bloom development. J. Food Eng. 2015, 146, 172–181. [Google Scholar] [CrossRef]
- Duong, Q.; Purgianto, A.; Maleky, F. Dynamics of moisture diffusivity in solid triacylglycerol matrices. Food Res. Int. 2015, 75, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Paluri, S.; Heldman, D.R.; Maleky, F. Effects of Structural Attributes and Phase Ratio on Moisture Diffusion in Crystallized Lipids. Cryst. Growth Des. 2017, 17, 4661–4669. [Google Scholar] [CrossRef]
- Tarabukina, E.; Jego, F.; Haudin, J.-M.; Navard, P.; Peuvrel-Disdier, E. Effect of Shear on the Rheology and Crystallization of Palm Oil. J. Food Sci. 2009, 74, E405–E416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, N.C.; Block, J.M.; Marangoni, A.G. Critical laminar shear-temperature effects on the nano- and mesoscale structure of a model fat and its relationship to oil binding and rheological properties. Faraday Discuss. 2012, 158, 171. [Google Scholar] [CrossRef]
- Maleky, F.; McCarthy, K.L.; McCarthy, M.J.; Marangoni, A.G. Effect of Cocoa Butter Structure on Oil Migration. J. Food Sci. 2012, 77, E74–E79. [Google Scholar] [CrossRef]
- Wang, H.; Maleky, F. Effects of cocoa butter triacylglycerides and minor compounds on oil migration. Food Res. Int. 2018, 106, 213–224. [Google Scholar] [CrossRef]
- Stapley, A.G.F.; Tewkesbury, H.; Fryer, P.J. The effects of shear and temperature history on the crystallization of chocolate. J. Am. Oil Chem. Soc. 1999, 76, 677–685. [Google Scholar] [CrossRef]
- Mazzanti, G.; Guthrie, S.E.; Sirota, E.B.; Marangoni, A.G.; Idziak, S.H.J. Orientation and Phase Transitions of Fat Crystals under Shear. Cryst. Growth Des. 2003, 3, 721–725. [Google Scholar] [CrossRef]
- Loisel, C.; Lecq, G.; Ponchel, G.; Keller, G.; Ollivon, M. Fat Bloom and Chocolate Structure Studied by Mercury Porosimetry. J. Food Sci. 1997, 62, 781–788. [Google Scholar] [CrossRef]
- Rousseau, D. On the porous mesostructure of milk chocolate viewed with atomic force microscopy. LWT Food Sci. Technol. 2006, 39, 852–860. [Google Scholar] [CrossRef]
- Lencki, R.W.; Craven, R.J. Negative Pressure Effects during Pure Triacylglyerol Crystallization. Cryst. Growth Des. 2012, 12, 4981–4986. [Google Scholar] [CrossRef]
- Lencki, R.W.; Craven, R.J. Negative Pressure Induced Cavity Formation During Cocoa Butter Crystallization. J. Am. Oil Chem. Soc. 2013, 90, 1509–1516. [Google Scholar] [CrossRef]
- Paluri, S.; Maleky, F.; Heldman, D.R. Development of a structure-based model for moisture diffusion in multiphase lipid networks. J. Food Eng. 2017, 214, 60–68. [Google Scholar] [CrossRef]
- Sofjan, R.P.; Hartel, R.W. Effects of overrun on structural and physical characteristics of ice cream. Int. Dairy J. 2004, 14, 255–262. [Google Scholar] [CrossRef]
- Nguyen, S.-T. Effect of pore shape on the effective behavior of viscoelastic porous media. Int. J. Solids Struct. 2017, 125, 161–171. [Google Scholar] [CrossRef]
- Verstringe, S.; Danthine, S.; Blecker, C.; Dewettinck, K. Influence of a commercial monoacylglycerol on the crystallization mechanism of palm oil as compared to its pure constituents. Food Res. Int. 2014, 62, 694–700. [Google Scholar] [CrossRef]
- Brun, M.; Delample, M.; Harte, E.; Lecomte, S.; Leal-Calderon, F. Stabilization of air bubbles in oil by surfactant crystals: A route to produce air-in-oil foams and air-in-oil-in-water emulsions. Food Res. Int. 2015, 67, 366–375. [Google Scholar] [CrossRef]
- Shi, X.; Maleky, F. Effects of external shear forces on crystallisation kinetics of model fat blends. Int. J. Food Sci. Technol. 2015, 50, 2255–2263. [Google Scholar] [CrossRef]
- Fredrick, E.; Foubert, I.; Van De Sype, J.; Dewettinck, K. Influence of Monoglycerides on the Crystallization Behavior of Palm Oil. Cryst. Growth Des. 2008, 8, 1833–1839. [Google Scholar] [CrossRef]
- Smith, P.R.; Cebula, D.J.; Povey, M.J.W. The effect of lauric-based molecules on trilaurin crystallization. J. Am. Oil Chem. Soc. 1994, 71, 1367–1372. [Google Scholar] [CrossRef]
- Charbonnet, G.H.; Singleton, W.S. Thermal properties of fats and oils. J. Am. Oil Chem. Soc. 1947, 24, 140–142. [Google Scholar] [CrossRef]
- Wille, R.L.; Lutton, E.S. Polymorphism of cocoa butter. J. Am. Oil Chem. Soc. 1966, 43, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Schlichter, J.; Mayer, I.; Sarig, S.; Garti, N. The Influence of Emulsifiers on the Crystal Properties of Tristearin and Trilaurin. Powder Diffr. 1988, 3, 19–22. [Google Scholar] [CrossRef]
- Maleky, F.; Marangoni, A.G. Process development for continuous crystallization of fat under laminar shear. J. Food Eng. 2008, 89, 399–407. [Google Scholar] [CrossRef]
- Kloek, W.; Van Vliet, T.; Walstra, P. Mechanical Properties of Fat Dispersions Prepared in a Mechanical Crystallizer. J. Texture Stud. 2005, 36, 544–568. [Google Scholar] [CrossRef]
- Acevedo, N.C.; Marangoni, A.G. Toward Nanoscale Engineering of Triacylglycerol Crystal Networks. Cryst. Growth Des. 2010, 10, 3334–3339. [Google Scholar] [CrossRef]
- Johansson, D.; Bergenståhl, B. Sintering of fat crystal networks in oil during post-crystallization processes. J. Am. Oil Chem. Soc. 1995, 72, 911–920. [Google Scholar] [CrossRef]
- Raeini, A.Q.; Bijeljic, B.; Blunt, M.J. Generalized network modeling: Network extraction as a coarse-scale discretization of the void space of porous media. Phys. Rev. E 2017, 96, 013312. [Google Scholar] [CrossRef] [Green Version]
- Indrawati, L.; Wang, Z.; Narsimhan, G.; Gonzalez, J. Effect of processing parameters on foam formation using a continuous system with a mechanical whipper. J. Food Eng. 2008, 88, 65–74. [Google Scholar] [CrossRef]
- Kalla, A.M.; Sahu, C.; Agrawal, A.K.; Bisen, P.; Chavhan, B.B.; Sinha, G. Development and performance evaluation of frustum cone shaped churn for small scale production of butter. J. Food Sci. Technol. 2016, 53, 2219–2226. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Guo, H.; Huang, Q.; Han, P.; Hou, Y.; Zou, W. Effect of stirring rate on microstructure and properties of microporous mullite ceramics. J. Mater. Process. Technol. 2018, 261, 159–163. [Google Scholar] [CrossRef]
- Perez-Martınez, J.D.; Reyes-Hernandez, J.; Dibildox-Alvarado, E.; Toro-Vazquez, J.F. Physical Properties of Cocoa Butter/Vegetable Oil Blends Crystallized in a Scraped Surface Heat Exchanger. J. Am. Oil Chem. Soc. 2012, 89, 199–209. [Google Scholar] [CrossRef]
- Sok, R.M.; Knackstedt, M.A.; Sheppard, A.P.; Pinczewski, W.V.; Lindquist, W.B.; Venkatarangan, A.; Paterson, L. Direct and Stochastic Generation of Network Models from Tomographic Images; Effect of Topology on Residual Saturations. Transp. Porous Media 2002, 46, 345–371. [Google Scholar] [CrossRef]
- Bernabé, Y.; Li, M.; Tang, Y.-B.; Evans, B. Pore Space Connectivity and the Transport Properties of Rocks. Oil Gas Sci. Technol. 2016, 71, 50. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarski, K.; Bellot, J.C. Effect of Particle-Size Distribution and Particle Porosity Changes on Mass Transfer Kinetics. Acta Chromatogr. 2003, 13, 16. [Google Scholar]
- Øren, P.-E.; Bakke, S. Reconstruction of Berea sandstone and pore-scale modelling of wettability effects. J. Pet. Sci. Eng. 2003, 39, 177–199. [Google Scholar] [CrossRef]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Kloek, W.; Van Vliet, T.; Walstra, P. Large Deformation Behavior of Fat Crystal Networks. J. Texture Stud. 2005, 36, 516–543. [Google Scholar] [CrossRef]
- Aronhime, J.S.; Sarig, S.; Garti, N. Dynamic control of polymorphic transformation in triglycerides by surfactants: The button syndrome. J. Am. Oil Chem. Soc. 1988, 65, 1144–1150. [Google Scholar] [CrossRef]
- Silin, D.; Patzek, T. Pore space morphology analysis using maximal inscribed spheres. Phys. A: Stat. Mech. Appl. 2006, 371, 336–360. [Google Scholar] [CrossRef]
- Al-Kharusi, A.S.; Blunt, M.J. Network extraction from sandstone and carbonate pore space images. J. Pet. Sci. Eng. 2007, 56, 219–231. [Google Scholar] [CrossRef]
- Dong, H.; Blunt, M.J. Pore-network extraction from micro-computerized-tomography images. Phys. Rev. E 2009, 80, 036307. [Google Scholar] [CrossRef] [Green Version]
- Raeini, A. Pnflow 2019. Available online: https://github.com/aliraeini/pnflow (accessed on 10 December 2020).
- Schere, D.; Sherwood, B. VPython (Version 7.5.0) Programming Package. 2017. Available online: https://vpython.org (accessed on 10 December 2020).








| Sample | Crystal Size (μm2) * | SFC (%) * |
|---|---|---|
| CB RS | 8.0 A ± 0.5 | 74.3 A ± 1.0 |
| CB LS | 8.5 A ± 0.4 | 76.4 B, C ± 0.4 |
| CB/M RS | 13.3 B ± 2.6 | 75.9 C ± 0.9 |
| CB/M LS | 11.4 B ± 0.8 | 77.4 B ± 0.6 |
| TL RS | 18.3 a ± 1.4 | 98.2 a ± 0.1 |
| TL LS | 13.9 a ± 4.2 | 97.8 b ± 0.0 |
| TL/M RS | 13.6 a ± 1.1 | 94.1 c ± 0.6 |
| TL/M LS | 18.6 a ± 5.1 | 95.4 c ± 0.1 |
| Sample | Connectivity * | Pore Radius * | Throat Radius * | Void Fraction * |
|---|---|---|---|---|
| z | R43,p (μm) | R43,t (μm) | v | |
| CB RS | 0.39 A, B ± 0.20 | 50.8 A ± 2.8 | 34.4 A ± 3.2 | 0.114 A ± 0.029 |
| CB LS | 0.42 A ± 0.12 | 50.0 A ± 3.9 | 44.4 A ± 6.8 | 0.019 B ± 0.018 |
| CB/M RS | 0.10 B ± 0.05 | 40.2 A ± 8.0 | 32.4 A ± 4.5 | 0.022 B ± 0.011 |
| CB/M LS | 1.88 C ± 0.37 | 45.4 A ± 17.7 | 28.8 A ± 10.6 | 0.046 B ± 0.027 |
| TL RS | 1.87 a, b± 0.12 | 61.2 a ± 3.5 | 33.81 a ± 5.8 | 0.118 a, b± 0.012 |
| TL LS | 2.44 a ± 0.62 | 31.8 b ± 6.8 | 18.01 b ± 3.6 | 0.139 a ± 0.034 |
| TL/M RS | 1.92 a, b ± 0.10 | 57.3 a ± 2.9 | 36.02 a ± 1.8 | 0.120 a, b ± 0.006 |
| TL/M LS | 1.05 b ± 0.38 | 24.2 b ± 4.2 | 12.32 b ± 3.1 | 0.087 b ± 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howard, B.D.; Maleky, F. Quantification of Porous Properties of Shear Crystallized Lipids. Molecules 2022, 27, 631. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030631
Howard BD, Maleky F. Quantification of Porous Properties of Shear Crystallized Lipids. Molecules. 2022; 27(3):631. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030631
Chicago/Turabian StyleHoward, Brandon D., and Farnaz Maleky. 2022. "Quantification of Porous Properties of Shear Crystallized Lipids" Molecules 27, no. 3: 631. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030631