Effect of Ultrasound-Assisted Extraction of Carotenoids from Papaya (Carica papaya L. cv. Sweet Mary) Using Vegetable Oils
Abstract
:1. Introduction
2. Results and Discussion
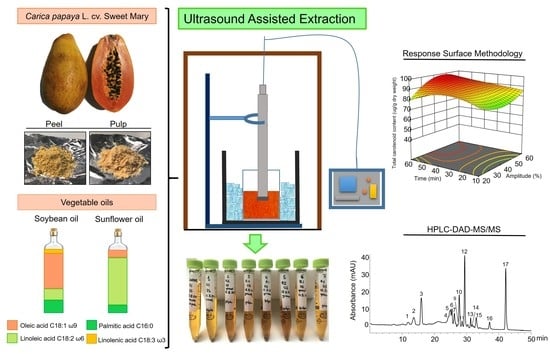
2.1. Carotenoid UAE from Papaya Pulp and Peel Using Vegetable Oils
2.2. Influence of Independent Variables on the Extraction Yields of Papaya Carotenoids and Carotenoid Esters
2.3. Optimization of UAE of Papaya Carotenoids with Vegetable Oils
3. Materials and Methods
3.1. Plant Material
3.2. Standards and Chemicals
3.3. Ultrasound-Assisted Extraction of Papaya Carotenoids
3.4. Experimental Design
3.5. HPLC Analysis of Carotenoids in Vegetable Extracts and Papaya Tissues
3.5.1. Carotenoid Extraction from Ultrasound Vegetable Oils Extract
3.5.2. Carotenoid Extraction from Control Vegetable Oils Extracts
3.5.3. Extraction of Carotenoids from Papaya Tissues
3.6. Carotenoid Analysis by HPLC-DAD
3.7. Carotenoid Analysis by Liquid Chromatography-Mass Spectrometry (LC-MS/MS (APCI+))
3.8. Determination of Total Carotenoid Content of Carotenoid- Oils Extracts
3.9. Color Determination of Carotenoid-Rich Vegetable Oils
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAO. Major Tropical Fruits: Preliminary Results. Rome. 2020. Available online: https://www.fao.org/3/cb6196en/cb6196en.pdf (accessed on 21 October 2021).
- Lara-Abia, S.; Lobo-Rodrigo, G.; Welti-Chanes, J.; Cano, M.P. Carotenoid and carotenoid ester profile and their deposition in plastids in fruits of new papaya (Carica papaya L.) varieties from the Canary Islands. Foods 2021, 10, 434. [Google Scholar] [CrossRef]
- Cabrera, J.A.; Ritter, A.; Raya, V.; Pérez, E.; Lobo, M.G. Papaya (Carica papaya L.) phenology under different agronomic conditions in the subtropics. Agriculture 2021, 11, 173. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Sivakumar, D.; Bello-Pérez, A.; Villanueva-Arce, R.; Hernández-López, M. A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Prot. 2013, 49, 8–20. [Google Scholar] [CrossRef]
- Vij, T.; Prashar, Y. A review on medicinal properties of Carica papaya Linn. Asian Pac. J. Trop. Dis. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Yap, J.Y.; Hii, C.L.; Ong, S.P.; Lim, K.H.; Abas, F.; Pin, K.Y. Effects of drying on total polyphenols content and antioxidant properties of Carica papaya leaves. J. Sci. Food Agric. 2020, 100, 2932–2937. [Google Scholar] [CrossRef]
- Teng, W.C.; Chan, W.; Suwanarusk, R.; Ong, A.; Ho, K.H.; Russell, B.; Koh, H.L. In vitro antimalarial evaluations and cytotoxicity investigations of Carica papaya leaves and carpaine. Nat. Prod. Commun. 2019, 14, 33–36. [Google Scholar] [CrossRef]
- Heena, D.; Sunil, T. Carica papaya: Potential implications in human health. Curr. Tradit. Med. 2019, 5, 321–336. [Google Scholar] [CrossRef]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Andreotti, M.; Spada, M.; Macchia, D.; Fais, S. Beneficial effects of fermented papaya Ppeparation (FPP®) supplementation on redox balance and aging in a mouse model. Antioxidants 2020, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Hu, S.; Park, Y.K.; Lee, J.Y. Health benefits of carotenoids: A role of carotenoids in the prevention of non-alcoholic fatty liver disease. Prev. Nutr. Food Sci. 2019, 24, 103. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yang, F.Y.; Lu, B.G.; Zhao, W.W.; Jiang, T.; Feng, L.; Chen, X.J.; Ming, R. Exploring the differential mechanisms of carotenoid biosynthesis in the yellow peel and red flesh of papaya. BMC Genom. 2019, 20, 49. [Google Scholar] [CrossRef] [Green Version]
- Hoover, D.G. Ultrasound. J. Food Saf. 2000, 65, 93–95. [Google Scholar]
- Mason, T.J. Power ultrasound in food processing-The way forward. In Ultrasound in Food Processing; Povey, M.J.W., Mason, T.J., Eds.; Blackie Academic and Professional: London, UK, 1998; pp. 105–126. [Google Scholar]
- Azmir, J.; Zaidul, I.S.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.; Ghafoor, K.; Norulaini, N.A.; Omar, A.K. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Sharma, S.R. Green chemistry, green solvents and alternative techniques in organic synthesis. Int. J. Chem. Phys. 2015, 4, 516–520. [Google Scholar]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Nour, V.; Corbu, A.R.; Rotaru, P.; Karageorgou, I.; Lalas, S. Effect of carotenoids, extracted from dry tomato waste, on the stability and characteristics of various vegetable oils. Grasas Aceites 2018, 69, e238. [Google Scholar] [CrossRef] [Green Version]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Lara-Abia, S.; Gomez-Maqueo, A.; Welti-Chanes, J.; Cano, M.P. High hydrostatic pressure-assisted extraction of carotenoids from papaya (Carica papaya L. cv. Maradol) tissues using soybean and sunflower oil as potential green solvents. Food Eng. Rev. 2021, 13, 660–675. [Google Scholar] [CrossRef]
- Song, J.; Yang, Q.; Huang, W.; Xiao, Y.; Li, D.; Liu, C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod. Process. 2018, 107, 104–112. [Google Scholar] [CrossRef]
- Chemat, F.; Perino-Issartier, S.; Loucif, L.; Elmaataoui, M.; Mason, T.J. Enrichment of edible oil with sea buckthorn by-products using ultrasound-assisted extraction. Eur. J. Lipid Sci. Technol. 2012, 114, 453–460. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Mahendrakar, N.S. Stability of carotenoids recovered from shrimp waste and their use as colorant in fish sausage. J. Food Sci. Technol. 2010, 47, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Cardoso, L.; Serrano, C.; Rodriguez, M.; de la Ossa, E.; Lubian, L. Extraction of carotenoids and fatty acids from microalgae using supercritical technology. AJAC 2012, 3, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Ordoñez-Santos, L.E.; Pinzon-Zarate, L.X.; Gonzalez-Salcedo, L.O. Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrason. Sonochem. 2015, 27, 560–566. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Y.; Liang, Z.; Wang, W. Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason. Sonochem. 2010, 17, 61–65. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Ramos-Parra, P.A.; García-Salinas, C.; Hernández-Brenes, C.; Díaz de la Garza, R.I. Folate levels and polyglutamylation profiles of papaya (Carica papaya cv. Maradol) during fruit development and ripening. J. Agric. Food Chem. 2013, 61, 3949–3956. [Google Scholar] [CrossRef]
- Plaza, L.; Colina, C.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem. 2012, 130, 591–597. [Google Scholar] [CrossRef]
- Mutsokoti, L.; Panozzo, A.; Musabe, E.T.; Van Loey, A.; Hendrickx, M. Carotenoid transfer to oil upon high pressure homogenisation of tomato and carrot based matrices. J. Funct. Foods 2015, 19, 775–785. [Google Scholar] [CrossRef] [Green Version]
- Cano, M.P.; Gomez-Maqueo, A.; Fernandez-Lopez, R.; Welti-Chanes, J.; Garcia-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods-A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef]
- Chandrika, U.G.; Jansz, E.R.; Wickramasinghe, S.N.; Warnasuriya, N.D. Carotenoids in yellow-and red-fleshed papaya (Carica papaya L.). J. Sci. Food Agric. 2003, 83, 1279–1282. [Google Scholar] [CrossRef]
- Hernandez-Brenes, C.; Ramos-Parra, P.A.; Jacobo-Velazquez, D.A.; Villarreal-Lara, R.; Diaz-de la Garza, R.I. High hydrostatic pressure processing as a strategy to increase carotenoid contents of tropical fruits. In Tropical and Subtropical Fruits: Flavors, Color, and Health Benefits; American Chemical Society: Washington, DC, USA, 2013; pp. 29–42. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Panel on Micronutrients—Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef] [Green Version]
- Breithaupt, D.E.; Schwack, W. Determination of free and bound carotenoids in paprika (Capsicum annuum L.) by LC/MS. Eur. Food Res. Technol. 2000, 211, 0052–0055. [Google Scholar]
- Melendez-Martinez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Steingass, C.B.; Mora, E.; Esquivel, P.; Carle, R. Carotenogenesis and physico-chemical characteristics during maturation of red fleshed papaya fruit (Carica papaya L.). Food Res. Int. 2011, 44, 1373–1380. [Google Scholar] [CrossRef]


| Peak | Rt (min) | Compound Identity | HPLC-DAD UV/Vis Absorption Maxima (nm) | %III/II | %Ab/AII | [M + H]+ m/z | HPLC/APCI+ MS Fragmentation Pattern (m/z) |
| 1 | 13.4 | (9Z)-α-cryptoxanthin | 412, 437, 466 | 0 | 0 | n.d. | n.d. |
| 2 | 13.9 | (all-E)-α-cryptoxanthin | (413), 435, 464 | 61 | n.c. | 553 | 535 [M + H − 18]+, 479, 461 [M + H − 92]+, 439 |
| 3 | 16.0 | (all-E)-β-cryptoxanthin | (426), 450, 476 | 18 | n.c. | 553 | 535 [M + H − H2O ]+, 461 [M + H − 92]+ |
| 4 | 23.7 | (9Z)-violaxanthin laurate | 388, 413, 436, 466 | 92 | 0 | 783 | 765 [M + H − H2O]+, [M + H − 18]+, 747 [M + H − 2H2O]+, [M + H − 18 − H2O]+, 565 [M + H − 12:0 − H2O]+, [M + H − 12:0 − 18]+ |
| 5 | 24.0 | (all-E)-lutein-3-O-myristate | 401, 426, 472 | 0 | 0 | n.d. | 533 [M + H − 228 − 18]+, 495 [M + H − 228 − 56]+, 459 [M + H − 228 − 92]+, 429, 441 |
| 6 | 24.3 | (all-E)-β-carotene | (428), 450, 476 | 16 | 0 | 537 | 457 [M + H − 80]+, 445 [M + H − 92]+, 400 [M + H − 137]+, 269 [M + H − 268]+, 177 [M + H − 360]+, 137 [M + H − 400]+ |
| 7 | 25.7 | (all-E)-antheraxanthin myristate palmitate | 421, 443, 467 | 31 | 0 | 1033 | 1015 [M + H − 18]+, 941 [M + H − 92]+, 805 [M + H − 14:0]+, 787 [M + H − 14:0 − 18]+, 771 [M + H − 16:0]+,759 [M + H − 16:0 − 18]+, 549 [M + H − 14:0 − 16:0]+, 531 [M + H − 14:0 − 16:0 − 18:0]+ |
| 8 | 26.1 | (all-E)-violaxanthin palmitate | 416, 441, 469 | nc | nc | 839 | 821 [M + H − 18]+, 803 [M + H − 18 − 18]+, 747 [M + H − 16:0]+, 729 [M + H − 92 − 18]+, 583 [M + H − 256]+, 565 [M + H − 18 − 16:0]+, 547 [M + H − 16:0 − 18 − 18]+ |
| 9 | 27.1 | (9Z)-neoxanthin dibutyrate | 327, 412, 436, 464 | 80 | 16 | 741 | 723 [M + H − 18]+, 653 [M + H − 4:0]+, 649 [M + H − 92]+, 635 [M + H − 4:0 − 18]+, 631 [M + H − 92 − 18]+, 565 [M + H − 4:0 − 4:0]+, 547 [M + H − 4:0 − 4:0 − 18]+ |
| 10 | 27.5 | (all-E)-β-cryptoxanthin caprate | 428, 450, 476 | nc | n.c. | 707 | 615 [M + H − 27]+, 535 [M + H − 100]+, 443 [M + H − 11]+, 442 [M + H − 16]+ |
| 11 | 28.6 | (all-E)-lutein dimyristate | 422, 446, 474 | 38 | 0 | n.d. | 761 M + H − 14:0]+, 669 [M + H − 92]+, 553 [M + H − 14:14:0]+ |
| 12 | 29.3 | (all-E)-β-cryptoxanthin laurate | 421, 451, 478 | 25 | 0 | 735 | 643 [M + H − 92]+, 535 [M + H − 12:0]+, 479 [M + H − 56 − 12:0]+, 443 [M + H − 92 − 12:0]+ |
| 13 | 31.2 | (all-E)-antheraxanthin laurate myristate | 418, 442, 470 | 33 | 0 | 977 | 959 [M + H − 18]+, 777 [M + H − 12:0]+, 749 [M + H − 14:0]+, 759 [M + H − 12:0 − 18]+, 731 [M + H − 14:0 − 18]+, 549 [M + H − 12:0 − 14:0]+, 531 [M + H − 12:0 − 14:0 − 18]+ |
| 14 | 32.0 | (all-E)-β-cryptoxanthin myristate | 424, 448, 476 | 9 | 0 | 763 | 671 [M + H − 92]+, 535 [M + H − 14:0]+, 443 [M + H − 14:0 − 92]+ |
| 15 | 32.9 | (13Z)-lycopene isomer 2 | 442, 465, 493 | 0 | 0 | 537 | 481 [M + H − 42]+, 467 [M + H − 35]+, 455 [M + H − 100]+, 427 [M + H − 61]+, 413 [M + H − 88]+, 399 [M + H − 24]+, 387 [M + H − 42]+ |
| 16 | 36.9 | (9Z)-lycopene isomer 4 | 440, 465, 496 | 0 | 0 | 537 | 481 [M + H − 11]+, 467 [M + H − 32]+, 455 [M + H − 79]+, 427 [M + H − 48]+, 413 [M + H − 30]+, 399 [M + H − 42]+, 387 [M + H − 38]+ |
| 17 | 41.7 | (all-E)-lycopene | 418, 443, 471, 502 | 6 | 0 | 537 | 457 [M + H − 80]+, 413 [M + H − 124]+, 177 [M + H − 360]+, 137 [M + H − 400]+, 121 [M + H − 416]+ |
| Run 1 | Amplitude (%) | Time (min) | EtOH (%) | Carotenoid Content (μg/g Vegetable Oil) 2 | |||
|---|---|---|---|---|---|---|---|
| Pulp | Peel | ||||||
| Soybean Oil | Sunflower Oil | Soybean Oil | Sunflower Oil | ||||
| 1 | 60 | 60 | 20 | 44.2 ± 1.8 b | 33.8 ± 1.0 a | 42.0 ± 1.1 b | 33.6 ± 0.7 a |
| 2 | 20 | 60 | 5 | 46.7 ± 0.7 d | 36.9 ± 1.1 c | 21.3 ± 0.3 b | 14.5 ± 0.3 a |
| 3 | 40 | 1 | 12.5 | 40.6 ± 1.0 c | 39.9 ± 1.4 c | 33.4 ± 1.0 b | 21.8 ± 0.6 a |
| 4 | 40 | 35 | 12.5 | 33.9 ± 1.0 c | 18.2 ± 0.5 a | 47.2 ± 0.7 d | 26.8 ± 0.4 b |
| 5 | 74 | 35 | 12.5 | 50.3 ± 1.7 c | 42.7 ± 0.8 b | 42.0 ± 0.9 b | 35.8 ± 0.6 a |
| 6 | 10 | 35 | 12.5 | 41.5 ± 1.1 b | 26.7 ± 0.5 a | 41.0 ± 0.8 b | 27.4 ± 0.7 a |
| 7 | 20 | 10 | 5 | 43.1 ± 1.0 b | 28.6 ± 0.3 a | 40.1 ± 0.7 b | 27.8 ± 0.4 a |
| 8 | 20 | 10 | 20 | 54.9 ± 2.2 d | 44.4 ± 0.9 c | 34.9 ± 0.7 b | 22.2 ± 0.2 a |
| 9 | 60 | 10 | 20 | 58.7 ± 1.6 c | 56.0 ± 1.5 c | 33.6 ± 0.6 b | 13.3 ± 0.3 a |
| 10 | 40 | 35 | 1 | 34.4 ± 1.1 c | 27.2 ± 0.5 b | 39.1 ± 1.3 c | 22.6 ± 0.7 a |
| 11 | 40 | 35 | 25 | 49.4 ± 1.2 c | 38.5 ± 0.8 b | 40.3 ± 1.1 b | 19.5 ± 0.2 a |
| 12 | 40 | 35 | 12.5 | 33.8 ± 0.7 b | 23.4 ± 0.7 a | 45.8 ± 1.4 c | 24.9 ± 0.6 a |
| 13 | 60 | 10 | 5 | 57.4 ± 1.6 b | 40.6 ± 0.7 a | 45.5 ± 1.1 a | 38.2 ± 1.5 a |
| 14 | 60 | 60 | 5 | 44.6 ± 1.5 ab | 46.5 ± 1.4 b | 43.9 ± 0.8 ab | 39.3 ± 0.5 a |
| 15 | 20 | 77 | 20 | 12.9 ± 0.7 b | 5.6 ± 0.1 a | 52.0 ± 0.9 d | 31.9 ± 0.6 c |
| 16 | 40 | 35 | 12.5 | 29.2 ± 1.1 a | 25.6 ± 0.6 a | 47.2 ± 1.4 b | 26.0 ± 0.3 a |
| C | - | - | - | 19.5 ± 0.6 b | 20.1 ± 0.7 b | 10.1 ± 0.2 a | 9.4 ± 0.2 a |
| Variables | Factors | Levels | |||||
| −1 | 1 | ||||||
| Amplitude (%) | A | 20 | 60 | ||||
| Time (min) | B | 10 | 60 | ||||
| Ethanol in Solvent (%) | C | 5 | 20 | ||||
| Statistical Data 1 | Soybean Oil | Sunflower Oil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pulp Extracts | Peel Extracts | Pulp Extracts | Peel Extracts | |||||||||
| Regression Coefficient | F-Value | p-Value | Regression Coefficient | F-Value | p-Value | Regression Coefficient | F-Value | p-Value | Regression Coefficient | F-Value | p-Value | |
| Model | 32.38 | 21.65 | 0.045 | 45.76 | 93.04 | 0.011 | 23.00 | 9.13 | 0.007 | 25.77 | 20.57 | 0.001 |
| A-Amplitude | 3.57 | 1.98 | 0.295 | 1.42 | 3.96 | 0.185 | 6.02 | 17.42 | 0.006 | 3.16 | 26.61 | 0.002 |
| B-Time | −7.19 | 0.11 | 0.769 | 1.68 | 58.92 | 0.017 | −5.84 | 13.87 | 0.010 | 2.07 | 9.69 | 0.021 |
| C-EtOH | 0.51 | 12.32 | 0.073 | 1.23 | 4.25 | 0.175 | 0.41 | 0.08 | 0.785 | −1.94 | 10.21 | 0.019 |
| AB | 0.88 | 0.17 | 0.723 | 0.73 | 10.98 | 0.080 | 1.65 | 0.90 | 0.380 | 3.51 | 22.43 | 0.003 |
| AC | 1.90 | 3.52 | 0.202 | −5.05 | 283.13 | 0.004 | 1.87 | 1.03 | 0.350 | −4.98 | 40.45 | 0.001 |
| BC | −5.40 | 26.79 | 0.035 | 6.07 | 420.01 | 0.002 | −9.28 | 28.25 | 0.002 | 4.89 | 43.64 | 0.001 |
| A2 | 6.13 | 29.48 | 0.032 | −1.52 | 50.79 | 0.019 | 4.53 | 7.50 | 0.034 | 2.36 | 11.32 | 0.015 |
| B2 | 0.81 | 16.22 | 0.057 | −3.21 | 46.56 | 0.021 | 4.50 | 5.76 | 0.053 | 0.10 | 0.02 | 0.904 |
| C2 | 5.05 | 14.34 | 0.063 | −2.16 | 91.41 | 0.011 | 4.33 | 6.81 | 0.040 | −1.43 | 4.16 | 0.088 |
| Lack of fit | 13.21 | 0.072 | 9.41 | 0.092 | 2.20 | 0.336 | 7.22 | 0.125 | ||||
| R2 | 0.993 | 0.998 | 0.932 | 0.969 | ||||||||
| Adjusted R2 | 0.947 | 0.988 | 0.830 | 0.922 | ||||||||
| Adequate precision | 18.235 | 40.604 | 12.210 | 14.847 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Abia, S.; Welti-Chanes, J.; Cano, M.P. Effect of Ultrasound-Assisted Extraction of Carotenoids from Papaya (Carica papaya L. cv. Sweet Mary) Using Vegetable Oils. Molecules 2022, 27, 638. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030638
Lara-Abia S, Welti-Chanes J, Cano MP. Effect of Ultrasound-Assisted Extraction of Carotenoids from Papaya (Carica papaya L. cv. Sweet Mary) Using Vegetable Oils. Molecules. 2022; 27(3):638. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030638
Chicago/Turabian StyleLara-Abia, Sara, Jorge Welti-Chanes, and M. Pilar Cano. 2022. "Effect of Ultrasound-Assisted Extraction of Carotenoids from Papaya (Carica papaya L. cv. Sweet Mary) Using Vegetable Oils" Molecules 27, no. 3: 638. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030638