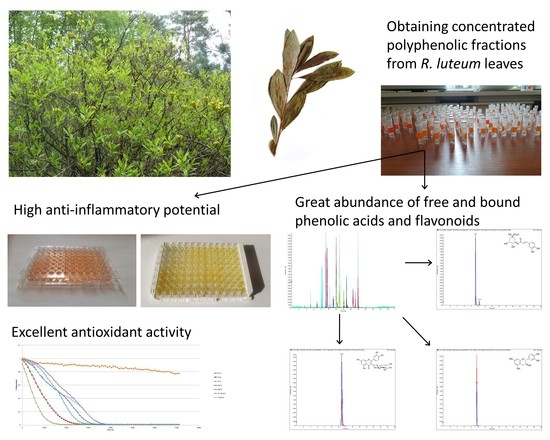
LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron luteum and Their Anti-Inflammatory and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profile of Polyphenolic Fractions
2.2. Antioxidant and Anti-Inflammatory Activity of Polyphenolic Fractions
3. Materials and Methods
3.1. Chemicals and Apparatus
3.2. Plant Material
3.3. Sample Preparation
3.4. LC-ESI(−)-MS/MS Analysis
3.5. Inhibition of Lipoxygenase (LOX) Activity
3.6. Inhibition of Cyclooxygenase (COX) Activity
3.7. Inhibition of Hyaluronidase Activity
3.8. ORAC
3.9. ABTS
3.10. Metal Chelating Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qiang, Y.; Zhou, B.; Gao, K. Chemical constituents of plants from the genus Rhododendron. Chem. Biodivers. 2011, 8, 1958–1967. [Google Scholar] [CrossRef]
- He, Y.C.; Yao, Y.M.; Xue, Q.W.; Fang, X.; Liang, S. Anti-rheumatoid arthritis potential of diterpenoid fraction derived from Rhododendron molle fruits. Chin. J. Nat. Med. 2021, 19, 181–187. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Sahin, K. Honey shows potent inhibitory activity against the bovine testes hyaluronidase. J. Enzyme Inhib. Med. Chem. 2016, 31, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Poomanee, W.; Khunkitti, W.; Chaiyana, W.; Intasai, N.; Lin, W.C.; Lue, S.C.; Leelapornpisid, P. Multifunctional biological properties and phytochemical constituents of Mangifera indica L. seed kernel extract for preventing skin aging. Toxicol. Res. 2021, 37, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Tekulu, G.H.; Hiluf, T.; Brhanu, H.; Araya, E.M.; Bitew, H.; Haile, T. Anti-inflammatory and anti-nociceptive property of Capparis tomentosa Lam. root extracts. J. Ethnopharmacol. 2020, 253, 112654. [Google Scholar] [CrossRef] [PubMed]
- Synoweć, J.; Pogorzelczyk, K.; Robakowska, M.; Nadolny, K. Następstwa stosowania ogólnodostępnych niesteroidowych leków przeciwzapalnych ( NLPZ ) [The consequences of using widely available non-steroidal anti-inflammatory drugs (NSAIDs)]. Medycyna Rodzinna 2018, 21, 281–291. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Sieniawska, E.; Sinan, K.I.; Nancy Picot-Allain, M.C.; Yerlikaya, S.; Cengiz Baloglu, M.; Altunoglu, Y.C.; Senkardes, I.; Rengasamy, K.R.; Zengin, G. Utilisation of Rhododendron luteum Sweet bioactive compounds as valuable source of enzymes inhibitors, antioxidant, and anticancer agents. Food Chem. Toxicol. 2020, 135, 111052. [Google Scholar] [CrossRef]
- Olech, M.; Łyko, L.; Nowak, R. Influence of accelerated solvent extraction conditions on the LC-ESI-MS/MS polyphenolic profile, triterpenoid content, and antioxidant and anti-lipoxygenase activity of rhododendron luteum sweet leaves. Antioxidants 2020, 9, 822. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kılıç, Ö. Evaluation of antioxidant capacity of endemic plant Marrubium astracanicum subsp. macrodon: Identification of its phenolic contents by using HPLC-MS/MS. Nat. Prod. Res. 2019, 33, 1975–1979. [Google Scholar] [CrossRef]
- Szewczyk, K.; Pietrzak, W.; Klimek, K.; Miazga-Karska, M.; Firlej, A.; Flisiński, M.; Grzywa-Celińska, A. Flavonoid and Phenolic Acids Content and In Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. Int. J. Mol. Sci. 2021, 22, 6219. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.K.; Towers, G.H.N. The identification, by chromatography, of plant phenolic acids. Arch. Biochem. Biophys. 1960, 87, 125–128. [Google Scholar] [CrossRef]
- Olech, M.; Pietrzak, W.; Nowak, R. Characterization of free and bound phenolic acids and flavonoid aglycones in Rosa rugosa Thunb. leaves and achenes using LC-ESI-MS/MS-MRM methods. Molecules 2020, 25, 1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.; Rezk, A.; Said, I.H.; Von Glasenapp, V.; Smith, R.; Ullrich, M.S.; Schepker, H.; Kuhnert, N. Comparison of the polyphenolic profile and antibacterial activity of the leaves, fruits and flowers of Rhododendron ambiguum and Rhododendron cinnabarinum. BMC Res. Notes 2017, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, L.Y.; Wang, S.L.; Niu, X.Y. Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant. Physiol. Biochem. 2016, 104, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.G.; Hong, K.; Tang, M.; Tang, J.; Liu, L.X.; Gao, G.F.; Shen, Z.J.; Zhang, X.M.; Yi, Y. Untargeted metabolite profiling of petal blight in field-grown Rhododendron agastum using GC-TOF-MS and UHPLC-QTOF-MS/MS. Phytochemistry 2021, 184, 112655. [Google Scholar] [CrossRef]
- Grimbs, A.; Shrestha, A.; Rezk, A.S.D.; Grimbs, S.; Hakeem Said, I.; Schepker, H.; Hütt, M.T.; Albach, D.C.; Brix, K.; Kuhnert, N.; et al. Bioactivity in rhododendron: A systemic analysis of antimicrobial and cytotoxic activities and their phylogenetic and phytochemical origins. Front. Plant. Sci. 2017, 8, 551. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Hakeem, I.; Grimbs, A.; Thielen, N.; Lansing, L.; Schepker, H.; Kuhnert, N. Determination of hydroxycinnamic acids present in Rhododendron species. Phytochemistry 2017, 144, 216–225. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Nikolaev, V.M.; Chirikova, N.K. Sagan dalya tea, a new “old” probable adaptogenic drug: Metabolic characterization and bioactivity potentials of rhododendron adamsii leaves. Antioxidants 2021, 10, 863. [Google Scholar] [CrossRef]
- Rateb, M.E.; Hassan, H.M.; Arafa, E.-S.A.; Jaspars, M.; Ebel, R. Decorosides A and B, cytotoxic flavonoid glycosides from the leaves of Rhododendron decorum. Nat. Prod. Commun. 2014, 9, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Razgonova, M.; Zakharenko, A.; Ercisli, S.; Grudev, V.; Golokhvast, K. Comparative analysis of far east sikhotinsky rhododendron (Rh. sichotense) and East Siberian Rhododendron (Rh. adamsii) using supercritical CO2-extraction and HPLC-ESI-MS/MS spectrometry. Molecules 2020, 25, 3774. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Prakash, D.; Upadhyay, G.; Singh, B.N.; Dhakarey, R.; Sandeep, K.; Singh, K.K. Free-radical scavenging activities of Himalayan rhododendrons. Curr. Sci. 2007, 92, 526–532. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Y.; Li, Z.Y.; Zheng, Y.; Chen, Y.; Zhou, Z.H.; Jin, J. The attenuation of chlorogenic acid on oxidative stress for renal injury in streptozotocin-induced diabetic nephropathy rats. Arch. Pharm. Res. 2016, 39, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Porto, G.; Cabrita, E.J.; Marques, M.M.B.; Fernandes, E. Inhibition of LOX by flavonoids: A structure-activity relationship study. Eur. J. Med. Chem. 2014, 72, 137–145. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Antioxidant Role of Catechin in Health and Disease. In Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 267–271. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Liu, Z.; Yang, K.; Chen, Z.; Cheng, Q.; Wu, L. The Versatile Effects of Dihydromyricetin in Health. Evid.-Based Complement. Altern. Med. 2017, 2017, 1053617. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.A.; Paulhill, K.J.; Davidson, L.A.; Lupton, J.R.; Taddeo, S.S.; Hong, M.Y.; Carroll, R.J.; Chapkin, R.S.; Turner, N.D. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J. Nutr. 2009, 139, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, F.; Liu, X.; Liu, J.; Li, D. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4–MyD88-mediated NF-κB and MAPK signaling pathways. Phyther. Res. 2019, 33, 756–767. [Google Scholar] [CrossRef]
- Maiga, A.; Malterud, K.E.; Diallo, D.; Paulsen, B.S. Antioxidant and 15-lipoxygenase inhibitory activities of the Malian medicinal plants Diospyros abyssinica (Hiern) F. White (Ebenaceae), Lannea velutina A. Rich (Anacardiaceae) and Crossopteryx febrifuga (Afzel) Benth. (Rubiaceae). J. Ethnopharmacol. 2006, 104, 132–137. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, R.E.; Isla, M.I.; De, L.; Vildoza, E.; Quispe, C.; Schmeda-Hirschmann, G.; Alberto, M.R. Inhibition of arachidonic acid metabolism by the Andean crude drug Parastrephia lucida (Meyen) Cabrera. J. Ethnopharmacol. 2013, 150, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.L.; Pinto, M.D.C.; Macias, P. Lipoxygenase inhibition by red wine phenolics compounds. J. Food Biochem. 2011, 35, 542–555. [Google Scholar] [CrossRef]
- Ooh, K.F.; Ong, H.C.; Wong, F.C.; Chai, T.T. HPLC profiling of phenolic acids and flavonoids and evaluation of anti-lipoxygenase and antioxidant activities of aquatic vegetable Limnocharis flava. Acta Pol. Pharm.-Drug Res. 2015, 72, 973–979. [Google Scholar]
- Nisar, M.; Ali, S.; Muhammad, N.; Gillani, S.N.; Shah, M.R.; Khan, H.; Francesco, M. Antinociceptive and anti-inflammatory potential of Rhododendron arboreum bark. Toxicol. Ind. Health 2014, 32, 1–6. [Google Scholar] [CrossRef]
- Matczak, M.; Marchelak, A.; Michel, P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Sorbus domestica L. leaf extracts as functional products: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. J. Funct. Foods 2018, 40, 207–218. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of Cosmeceutical Potentials of Selected Mushroom Fruitbody Extracts Through Evaluation of Antioxidant, Anti-Hyaluronidase and Anti-Tyrosinase Activity. J 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.M.; Park, J.; Cho, J.Y. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef] [Green Version]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Marchelak, A.; Sopinska, M.; Ciszewski, P.; Nowak, P.; Olszewska, M.A. Polyphenol-rich extracts from cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules 2018, 23, 2472. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, K.; Wróbel, D.; Żmudzki, P.; Podolak, I. Anti-inflammatory activity of saponins from roots of Impatiens parviflora DC. Nat. Prod. Res. 2020, 34, 1581–1585. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, C.; Lissi, E.A. Reaction of 2,2′-azinobis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) derived radicals with hydroperoxides. Kinetics and mechanism. Int. J. Chem. Kinet. 1998, 30, 565–570. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiliç, I.; Yeşiloǧlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and Anti-inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-state Fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lee, S.H.; Tai, B.H.; Jang, H.D.; Kim, Y.H. Antioxidant and anti-osteoporosis activities of chemical constituents of the stems of Zanthoxylum piperitum. Molecules 2018, 23, 457. [Google Scholar] [CrossRef] [Green Version]
- Olech, M.; Nowak, R.; Ivanova, D.; Tashev, A.; Boyadzhieva, S.; Kalotova, G.; Angelov, G.; Gawlik-Dziki, U. LC-ESI-MS/MS-MRM profiling of polyphenols and antioxidant activity evaluation of junipers of different origin. Appl. Sci. 2020, 10, 8921. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Nowak, R.; Lemieszek, M.K.; Rzeski, W.; Gawlik-Dziki, U.; Szpakowska, N.; Kaczyński, Z. Promising potential of crude polysaccharides from Sparassis crispa against colon cancer: An in vitro study. Nutrients 2021, 13, 161. [Google Scholar] [CrossRef]
- Yahaya, Y.A.; Don, M.M. Evaluation of Trametes lactinea extracts on the inhibition of hyaluronidase, lipoxygenase and xanthine oxidase activities in Vitro. J. Phys. Sci. 2012, 23, 1–15. [Google Scholar]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubínski, K.; Załuski, D.; Nowak, R. Polysaccharide-rich fractions from Rosa rugosa Thunb.-composition and chemopreventive potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.T.; Lee, H.L.; Chiang, S.H.; Lin, F.I.; Chang, C.Y. Antioxidant Properties of the Extracts from Different Parts of Broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar] [CrossRef]


| RL-M | FR A | FR B | FR C | FR AA | FR BB | |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| gallic | 0.267 ± 0.001 | 3.383 ± 0.000 | 8.200 ± 0.384 | 0 | 3.376 ± 0.059 | 0.777 ± 0.003 |
| protocatechuic | 0.397 ± 0.002 | 19.141 ± 0.154 | 5.160 ± 0.170 | 0 | 4.250 ± 0.026 | 4.730 ± 0.048 |
| 4-hydroxybenzoic | BQL | BQL | BQL | 0 | BQL | BQL |
| gentisic | 0.016 ± 0.001 | 0.667 ± 0.002 | 2.248 ± 0.056 | BQL | 0.335 ± 0.003 | 0.162 ± 0.007 |
| caffeic | BQL | BQL | 33.056 ± 0.042 | 0 | 0 | 0 |
| vanillic | 0 | 0 | BQL | 0 | 0 | BQL |
| p-coumaric | 0.087 ± 0.003 | 5.891 ± 0.155 | BQL | 146.840 ± 1.485 | 5.293 ± 0.181 | 1.551 ± 0.074 |
| ferulic | BQL | 40.375 ± 1.055 | BQL | 0 | BQL | BQL |
| 5-O-caffeoylquinic | 18.546 ± 0.093 | 0 | 0 | 0 | 11.191 ± 0.526 | 1.258 ± 0.057 |
| Flavonoid aglycones | ||||||
| laricitrin | BQL | 0 | 0 | 0 | 0 | BQL |
| dihydromyricetin | 4.032 ± 0.086 | 102.912 ± 4.720 | 29.120 ± 2.712 | 1.595 ± 0.007 | 91.923 ± 4.678 | 2.637 ± 0.085 |
| catechin | 6.081 ± 0.043 | 51.278 ± 0.402 | 26.720 ± 0.904 | 0 | 55.678 ± 0.155 | 6.869 ± 0.184 |
| naringenin | 0.588 ± 0.005 | 0 | 0 | 0 | BQL | BQL |
| taxifolin | 0.019 ± 0.000 | 2.675 ± 0.044 | BQL | 0 | 0.928 ± 0.031 | BQL |
| myricetin | 1.377 ± 0.012 | BQL | BQL | 0 | 15.293 ± 0.802 | 56.404 ± 1.589 |
| luteolin | BQL | BQL | BQL | 0 | BQL | BQL |
| eriodictyol | 0.113 ± 0.001 | 0.844 ± 0.014 | 0.637 ± 0.030 | 0 | 0.480 ± 0.005 | 0.188 ± 0.011 |
| quercetin | 1.609 ± 0.011 | 23.722 ± 0.603 | 34.160 ± 0.340 | 29.210 ± 0.370 | 23.000 ± 0.979 | 84.270 ± 1.589 |
| 3-O-methylquercetin | 0.013 ± 0.000 | 0 | 0 | 0 | 0.304 ± 0.015 | 0.574 ± 0.001 |
| apigenin | BQL | BQL | BQL | 0 | BQL | BQL |
| kaempferol | BQL | BQL | BQL | BQL | BQL | BQL |
| Flavonoid glycosides | ||||||
| afzelin | 0.018 ± 0.002 | 0 | 0 | 0 | 0 | 0 |
| hyperoside | 9.044 ± 0.000 | 0 | 0 | 0 | 0 | 0 |
| isoquercetin | 8.980 ± 0.086 | 0 | 0 | 0 | 0 | 0 |
| vitexin | BQL | 0 | 0 | 0 | 0 | 0 |
| isovitexin | BQL | 0 | 0 | 0 | 0 | 0 |
| quercitrin | 1.822 ± 0.024 | 0 | 0 | 0 | 0 | 0 |
| naringenin 7-O-glucoside | BQL | 0 | 0 | 0 | 0 | 0 |
| Sample | COX 1 Inhibitory Activity IC50 (µg/mL) | COX 2 Inhibitory Activity IC50 (µg/mL) |
|---|---|---|
| RL-M | 311.18 ± 10.95 | 608.09 ± 14.78 |
| FR A | 0 | 0 |
| FR B | 0 | 0 |
| FR C | 0 | 0 |
| FR AA | 0 | 53.40 ± 5.07 |
| FR BB | 0 | 0 |
| ASA | 14.80 ± 0.27 | 35.0 ± 2.93 |
| Sample | Lipoxygenase IC50 (mg DE/mL) | Hyaluronidase IC50 (µg DE/mL) |
|---|---|---|
| RL-M | 2.27 ± 0.01 | 305.21 ± 3.21 |
| FR A | 1.11 ± 0.07 | >1000 |
| FR B | 0.33 ± 0.01 | 97.44 ± 4.98 |
| FR C | 0.40 ± 0.01 | 78.76 ± 2.09 |
| FR AA | 2.54 ± 0.14 | 429.07 ± 31.08 |
| FR BB | 2.96 ± 0.06 | >1000 |
| EGCG | n. a. | 276.50 ± 1.34 |
| ASA | 2.12 ± 0.13 | n. a. |
| Sample | ABTS (g Trolox/g) | ORAC (g Trolox/g) | Chelating Power (mg Na2EDTA/g) |
|---|---|---|---|
| RL-M | 4.67 ± 0.00 | 5.76 ± 0.03 | 4.73 ± 0.33 |
| FR A | 3.03 ± 0.04 | 175.32 ± 9.35 | 128.54 ± 10.444 |
| FR B | 4.18 ± 0.04 | 221.81 ± 20.19 | 292.36 ± 39.72 |
| FR C | 5.09 ± 0.23 | 91.19 ± 0.50 | 521.81 ± 26.22 |
| FR AA | 1.68 ± 0.01 | 107.14 ± 1.48 | 80.39 ± 6.35 |
| FR BB | 0.62 ± 0.03 | 40.87 ± 0.14 | 42.78 ± 0.44 |
| Sample | RL-M | FR A | FR B | FR C | FR AA | FR BB |
|---|---|---|---|---|---|---|
| Extraction Efficiency (%) | 31.18 | 0.64 | 0.25 | 0.20 | 1.35 | 2.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyko, L.; Olech, M.; Nowak, R. LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron luteum and Their Anti-Inflammatory and Antioxidant Activities. Molecules 2022, 27, 827. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030827
Łyko L, Olech M, Nowak R. LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron luteum and Their Anti-Inflammatory and Antioxidant Activities. Molecules. 2022; 27(3):827. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030827
Chicago/Turabian StyleŁyko, Lena, Marta Olech, and Renata Nowak. 2022. "LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron luteum and Their Anti-Inflammatory and Antioxidant Activities" Molecules 27, no. 3: 827. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27030827