Exploring the Antiparasitic Activity of Tris-1,3,4-Thiadiazoles against Toxoplasma gondii-Infected Mice
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.1.1. Synthesis of the Targeted Compounds
2.1.2. Spectroscopic Characterization of the Targeted Compounds
2.2. Acute Toxicity
2.3. Clinical Study


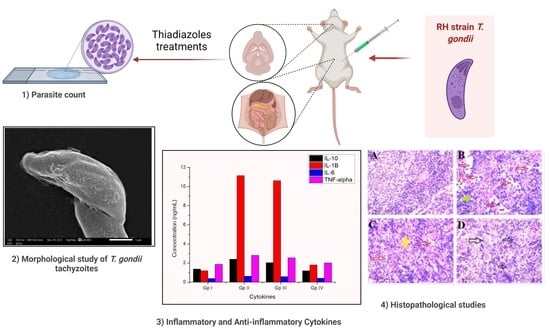
2.4. Parasitological Study
2.4.1. Parasite Count and Percent Reduction (%R)
2.4.2. Morphological Study of T. gondii Tachyzoites
2.5. Inflammatory and Anti-Inflammatory Cytokines in Infected and Treated Groups
2.6. Histopathological Study
2.6.1. Liver
2.6.2. Spleen
2.6.3. Brain
2.7. Molecular Docking
3. Materials and Methods
3.1. Drugs Synthesis and Analysis
3.1.1. General
3.1.2. Synthesis and Characterization of Diethyl 4,4′-[(1,3,4-Thiadiazol-2,5diyl)bis(sulfanediyl)]dibutanoate (2)
3.1.3. Synthesis and Characterization of 4,4′-[(1,3,4-Thiadiazol-2,5-diyl)bis(sulfanediyl)]dibutanehydrazide (3)
3.1.4. General Procedure for the Synthesis of Thiosemicarbazide Derivatives 4 and 5
3.1.5. General Procedure for the Synthesis of Thiadiazole Derivatives 6–7
3.2. In Vivo Acute Toxicity Study
- Group 1: Mice were given 100 μL normal saline (0.9%)
- Group 2: Mice were given 100 μL compound 6 (10 mg/kg) body mass index (low dose).
- Group 3: Mice were given 100 μL compound 6 (100 mg/kg) body mass index (high dose).
- Group 4: Mice were given 100 μL compound 7 (10 mg/kg) body mass index (low dose).
- Group 5: Mice were given 100 μL compound 7 (100 mg/kg) body mass index (high dose).

3.3. Parasite
3.4. Drugs Preparation
3.5. Animal Grouping and Experimental Design
- Group I: Healthy control, each mouse received 100 μL normal saline for seven days.
- Group II: Infected untreated control, each mouse received 100 μL normal saline (the vehicle of the used drugs) orally by gavage needle starting from the day of infection for seven days.
- Group III: Infected mice received 100 μL of compound 6 at a dose of 10 mg/kg/day orally by gavage needle starting from the day of infection for seven days.
- Group IV: Infected mice received 100 μL of compound 7 at a dose of 10 mg/kg/day orally by gavage needle starting from the day of infection for seven days.
- Group V: Infected mice received 100 μL of megazole at a dose of 10 mg/kg/day orally by gavage needle starting from the day of infection for seven days.

3.5.1. Clinical Research
3.5.2. Parasitological Study
Estimation of the Parasite Count
Parasite Percent Reduction (%R)
Morphological Study of T. gondii Tachyzoites
3.5.3. Inflammatory Biomarkers
3.5.4. Histopathological Study
3.6. Mode of Action of the Tested Compounds
Molecular Docking
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Montoya, J.G.; Liensefeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hoeta, I.; Olariu, T.T.; Jones, J.L.; Darabus, G. Epidemiological review of toxoplasmosis in human and animals in Romania. Parasitology 2014, 141, 225–311. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Dardé, M.L. Epidemiology of diagnostic for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfert, E.A.; Blader, I.J.; Wilson, E.H. Brains and Brawn: Toxoplasma infections of the central nervous system and skeletal muscle. Trends Parasitol. 2017, 33, 519–531. [Google Scholar] [CrossRef]
- Jensen, K.D.; Wang, Y.; Wojno, E.D.T.; Shastri, A.J.; Hu, K.; Cornel, L.; Boedec, E.; Ong, Y.C.; Chien, Y.H.; Hunter, C.A.; et al. Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe. 2011, 9, 472–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordue, D.G.; Monroy, F.; La Regina, M.; Dinarello, C.A.; Sibley, L.D. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 2001, 167, 4574–4584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.S.; Shin, J.H.; Yang, J.P.; Jung, B.K.; Lee, S.H.; Shin, E.H. Characteristics of infection immunity regulated by Toxoplasma gondii to maintain chronic infection in the brain. Front. Immunol. 2018, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serban, G. 2-Amino-1, 3, 4-thiadiazoles as prospective agents in trypanosomiasis and other parasitoses. Acta Pharm. 2020, 70, 259–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourido, S.; Zhang, C.; Lopez, M.S.; Tang, K.; Barks, J.; Wang, Q.; Wildman, S.A.; Shokat, K.M.; Sibley, L.D. Optimizing small molecule inhibitors of calcium-dependent protein kinase 1 to prevent infection by Toxoplasma gondii. J. Med. Chem. 2013, 56, 3068–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273. [Google Scholar] [CrossRef] [Green Version]
- Węglińska, L.; Bekier, A.; Dzitko, K.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Plech, T.; Paneth, P.; Paneth, A. 1,3,4-Thiadiazoles Effectively Inhibit Proliferation of Toxoplasma gondii. Cells 2021, 10, 1053. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Alam, M.S.; Hamid, H. 1,3,4-Thiadiazoles: A potent multi targeted pharmacological scaffold. Eur. J. Med. Chem. 2015, 92, 156–177. [Google Scholar] [CrossRef]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and its Derivatives: A Review on Recent Progress in Biological Activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef]
- Li, Q.; Ren, J.; Dong, F.; Feng, Y.; Gu, G.; Guo, Z. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013, 373, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Aouad, M.R.; Messali, M.; Rezki, N.; Ali, A.A.S.; Lesimple, A. Synthesis and characterization of some novel 1,2,4-triazoles, 1,3,4-thiadiazoles and Schiff bases incorporating imidazole moiety as potential antimicrobial agents. Acta Pharm. 2015, 65, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadi, A.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Al-Yahyawi, A.M.; Bardaweel, S.K.; Al-Blewi, F.F.; Aouad, M.R. Synthesis of novel 2,5-disubstituted-1,3,4-thiadiazoles clubbed 1,2,4-triazole, 1,3,4-thiadiazole, 1,3,4-oxadiazole and/or Schiff base as potential antimicrobial and antiproliferative agents. Molecules 2015, 20, 16048–16067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; Soliman, M.A.; Aouad, M.R.; Messali, M.; Rezki, N. Synthesis, Characterization and biological screening of novel 1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles bearing indole moiety. Org. Prep. Proced. Int. 2019, 51, 270. [Google Scholar] [CrossRef]
- Liesen, A.P.; de Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; de Araújo, J.M.; de Lima, J.G.; de Faria, A.R.; de Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.Y.; Shaaban, M.M.; Elwakil, B.H.; Hamed, M.T.; Rezki, N.; Aouad, M.R.; Zakaria, M.A.; Hagar, M. Anti-COVID-19 activity of some benzofused 1,2,3-triazolesulfonamide hybrids using in silico and in vitro analyses. Chemom. Intell. Lab. Syst. 2021, 217, 104421. [Google Scholar] [CrossRef]
- Mantenuto, S.; Lucarini, S.; De Santi, M.; Piersanti, G.; Brandi, G.; Favi, G.; Mantellini, F. One-pot synthesis of biheterocycles based on indole and azole scaffolds using tryptamines and 1,2-diaza-1,3-dienes as building blocks. Eur. J. Org. Chem. 2016, 9, 3193–3199. [Google Scholar] [CrossRef]
- Reddy, K.H.V.; Brion, J.-D.; Messaoudi, S.; Alami, M. Synthesis of biheterocycles based on quinolinone, chromone and coumarin scaffolds by palladium-catalyzed decarboxylative couplings. Org. Chem. 2016, 81, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Erşatır, M.; Yıldırım, M.; Giray, E.S.; Yalın, S. Synthesis and antiproliferative evaluation of novel biheterocycles based on coumarin and 2-aminoselenophene-3-carbonitrile unit. Mon. Für Chem.-Chem. Mon. 2020, 151, 625–636. [Google Scholar] [CrossRef]
- Tahghighi, A.; Babalouei, F. Thiadiazoles: The appropriate pharmacological scaffolds with leishmanicidal and antimalarial activities: A review. Iran. J. Basic Med. Sci. 2017, 20, 613. [Google Scholar] [PubMed]
- Kuo, J.-Z.; Chen, S.-Ê. Preparation of 2,5-dimercapto-1,3,4-thiadiazole and its derivatives. Acta Chim. Sin. 1963, 29, 62–63. [Google Scholar]
- Gaafar, M.R.; El-Mansoury, S.T.; Eissa, M.M.; Shalaby, T.I.; Younis, L.K.; Rashed, H.A. Effect of alginate nanoparticles on the immunogenicity of excretory-secretory antigens against acute toxoplasmosis in murine model. Acta Trop. 2022, 225, 106215. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Castro, B.E.; Reyes-García, J.G.; Valenzuela-Vargas, M.T.; Martínez-Gómez, F. Histopathology of murine toxoplasmosis under treatment with dialyzable leukocyte extract. Memórias Inst. Oswaldo Cruz 2017, 112, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Parlog, A.; Schlüter, D.; Dunay, I.R. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015, 37, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.A.; Ramos, G.A.; Zamora-Vélez, A.; Gallego-López, G.M.; Rocha-Roa, C.; Gómez-Marin, J.E.; Cortes, E. In vitro evaluation of new 4-thiazolidinones on invasion and growth of Toxoplasma gondii. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.S.; Eseola, A.O.; Plass, W.; Atolani, O.; Sugi, T.; Han, Y.; Batiha, G.E.S.; Kato, K.; Awakan, O.J.; Olaolu, T.D.; et al. Imidazole derivatives as antiparasitic agents and use of molecular modeling to investigate the structure–activity relationship. Parasitol. Res. 2020, 119, 1925–1941. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Elwakil, B.H.; Abu-Serie, M.M.; Aljohani, F.S.; Bekhit, A.A. Novel Synthesis of Titanium Oxide Nanoparticles: Biological Activity and Acute Toxicity Study. Bioinorg. Chem. Appl. 2021, 2021, 8171786. [Google Scholar] [CrossRef] [PubMed]
- Thiptara, A.; Kongkaew, W.; Bilmad, U.; Bhumibhamon, T.; Anan, S. Toxoplasmosis in piglets. Ann. N. Y. Acad. Sci. 2006, 1081, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Penido, M.L.O.; Nelson, D.L.; Vieira, L.Q.; Coelho, P.M.Z. Schistosomal activity of alkyl aminooctanethiosulfuric acids. Memórias Inst. Oswaldo Cruz. 1994, 89, 595–602. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Merwid-Ląd, A.; Wiatrak, B.; Danielewski, M.; Dzimira, S.; Szkudlarek, D.; Szczukowski, Ł.; Świątek, P.; Szeląg, A. Novel 1,3,4-Oxadiazole Derivatives of Pyrrolo [3, 4-d] Pyridazinone Exert Anti-Inflammatory Activity without Acute Gastrotoxicity in the Carrageenan-Induced Rat Paw Edema Test. J. Inflamm. Res. 2021, 14, 5739. [Google Scholar] [CrossRef] [PubMed]











| Liver (n = 10) | Spleen (n = 10) | Brain (n = 10) | |
|---|---|---|---|
| Group II Mean ± SD | 10.10 ± 1.20 | 5.40 ± 0.84 | 2.30 ± 0.67 |
| Group III (Cpd 6) Mean ± SD R1% | 6.60 ± 1.17 a,c,d 34.56 | 3.40 ± 0.96 a,c,d 37.03 | 1.10 ± 0.57 a,d 52.2 |
| Group IV (Cpd 7) Mean ± SD R2% | 3.50 ± 1.18 a,b,d 65.35 | 1.90 ± 0.57 a,b 64.81 | 0.40 ± 0.52 a 82.6 |
| Group V Mean ± SD R3% | 5.40 ± 0.84 a,b,c 46.53 | 2.60 ± 0.70 a,b 51.9 | 0.80 ± 0.63 a,b 65.3 |
| F p | 74.63 <0.001 | 27.51 <0.001 | 18.63 <0.001 |
| Mice Groups | TNF-ɑ (ng/mL) | IL-10 (ng/mL) | IL-6 (pg/mL) | IL-1B (ng/mL) |
|---|---|---|---|---|
| Group I | 1.87 | 1.37 | 393.87 | 1.20 |
| Group II | 2.81 | 2.41 | 626.81 | 11.14 |
| Group III | 2.55 | 2.05 | 579.26 | 10.63 |
| Group IV | 2.01 | 1.19 | 413.24 | 1.81 |
| Group V | 2.47 | 2.13 | 592.46 | 11.08 |
| Compounds | Affinity TgCDPK1 (kcal/mol) | Binding Features | Affinity TgROP18 (kcal/mol) | Binding Features |
|---|---|---|---|---|
| Megazol | −5.4 | ASN190, ARG143 (Hydrogen bond) | −5.0 | ALA359 (Hydrogen bond) |
| Compound 6 | −3.7 | GLY213, GLU178 (Hydrogen bond) | −4.6 | GLU300 (Hydrogen bond) |
| Compound 7 | −7.5 | HIS108 (Pi-Alkyl bond) | −5.5 | VAL266, LEU416, ALA359 (Pi-Alkyl bond) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, T.M.; Rezki, N.; Aouad, M.R.; Hagar, M.; Bakr, B.A.; Hamed, M.T.; Hassen, M.K.; Elwakil, B.H.; Moneer, E.A. Exploring the Antiparasitic Activity of Tris-1,3,4-Thiadiazoles against Toxoplasma gondii-Infected Mice. Molecules 2022, 27, 2246. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072246
Almutairi TM, Rezki N, Aouad MR, Hagar M, Bakr BA, Hamed MT, Hassen MK, Elwakil BH, Moneer EA. Exploring the Antiparasitic Activity of Tris-1,3,4-Thiadiazoles against Toxoplasma gondii-Infected Mice. Molecules. 2022; 27(7):2246. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072246
Chicago/Turabian StyleAlmutairi, Tahani M., Nadjet Rezki, Mohamed Reda Aouad, Mohamed Hagar, Basant A. Bakr, Moaaz T. Hamed, Maha Khairy Hassen, Bassma H. Elwakil, and Esraa Abdelhamid Moneer. 2022. "Exploring the Antiparasitic Activity of Tris-1,3,4-Thiadiazoles against Toxoplasma gondii-Infected Mice" Molecules 27, no. 7: 2246. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072246