Sustainable Recovery of Valuable Nanoporous Materials from High-Chlorine MSWI Fly Ash by Ultrasound with Organic Acids
Abstract
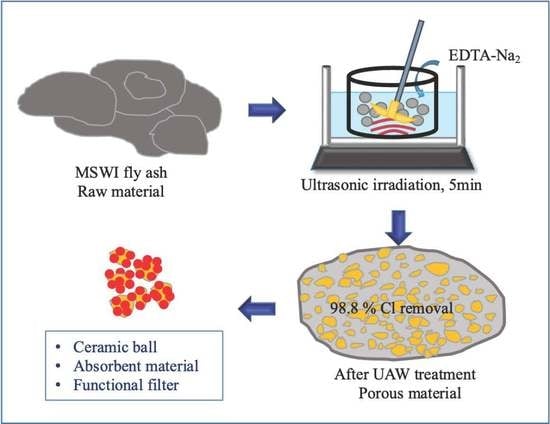
:1. Introduction
2. Results and Discussion
2.1. Physical and Chemical Properties of Raw MSWI Fly Ash
2.2. Results of Taguchi DOE Methodology
2.3. ANOVA Analysis of Variance
2.4. Effect of Experimental Parameters on Cl Removal
2.4.1. Effect of Acid Concentration (Factor A)
2.4.2. Effect of Ultrasonic Irradiation Time (Factor B)
2.4.3. Effect of S/L Ratio (Factor C)
2.4.4. Effect of Vertical Position (Factor D)
2.5. Characterization of Fly Ash before and after Treatment
2.6. Cl Removal Mechanism
2.7. Synergistic Effect of Multi-Frequency Ultrasound
3. Materials and Methods
3.1. Materials
3.2. Experiment Procedure
3.3. Design of Experiment by Taguchi Methodology
3.4. Calorimetry
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, M.; Xiang, J.; Hu, S.; Sun, L.-S.; Su, S.; Li, P.-S.; Sun, X.-X. Characterization of solid residues from municipal solid waste incinerator. Fuel 2004, 83, 1397–1405. [Google Scholar] [CrossRef]
- Ecke, H.; Sakanakura, H.; Matsuto, T.; Tanaka, N.; Lagerkvist, A. State-of-the-art treatment processes for municipal solid waste incineration residues in Japan. Waste Manag. Res. 2000, 18, 41–51. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Ai, H.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Clean. Prod. 2021, 15, 319. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, Y.; Bian, S.; Ko, J.H.; Li, S.F.Y.; Xu, Q. H2S adsorption by municipal solid waste incineration (MSWI) fly ash with heavy metals immobilization. Chemosphere 2018, 195, 40–47. [Google Scholar] [CrossRef]
- Tang, J.; Su, M.; Wu, Q.; Wei, L.; Wang, N.; Xiao, E.; Zhang, H.; Wei, Y.; Liu, Y.; Ekberg, C.; et al. Highly efficient recovery and clean-up of four heavy metals from MSWI fly ash by integrating leaching, selective extraction and adsorption. J. Clean. Prod. 2019, 234, 139–149. [Google Scholar] [CrossRef]
- Qiu, Q.; Jiang, X.; Lv, G.; Chen, Z.; Lu, S.; Ni, M.; Yan, J.; Deng, X. Adsorption of heavy metal ions using zeolite materials of municipal solid waste incineration fly ash modified by microwave-assisted hydrothermal treatment. Powder Technol. 2018, 335, 156–163. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chen, J.C. Resourcization and valorization of waste incineration fly ash for the synthesis of zeolite and applications. J. Environ. Chem. Eng. 2021, 9, 109549. [Google Scholar] [CrossRef]
- Koshy, N.; Singh, D.N. Fly ash zeolites for water treatment applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Jawad, A.H.; Malek, N.N.A.; Abdulhameed, A.S.; Razuan, R. Synthesis of Magnetic Chitosan-Fly Ash/Fe3O4 Composite for Adsorption of Reactive Orange 16 Dye: Optimization by Box–Behnken Design. J. Polym. Environ. 2020, 28, 1068–1082. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Depero, L.E.; Bontempi, E. Review of the reuse possibilities concerning ash residues from thermal process in a medium-sized urban system in Northern Italy. Sustainability 2020, 10, 4193. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Ye, T.; Wang, F.; Lan, Y. Utilization of washed MSWI fly ash as partial cement substitute with the addition of dithiocarbamic chelate. J. Environ. Manag. 2008, 88, 293–299. [Google Scholar] [CrossRef]
- Mangialardi, T. Disposal of MSWI fly ash through a combined washing-immobilisation process. J. Hazard. Mater. 2003, 98, 225–240. [Google Scholar] [CrossRef]
- Gau, S.H.; Wu, C.W.; Sun, C.J. Washing pretreatment to improve the quality during the regeneration of MSWI fly ash. In Proceedings of the Third International Conference on Waste Management and Technology, Beijing, China, 5–7 November 2008. [Google Scholar]
- Wang, X.; Gao, M.; Wang, M.; Wu, C.; Wang, Q.; Wang, Y. Chloride removal from municipal solid waste incineration fly ash using lactic acid fermentation broth. J. Waste Manag. 2021, 130, 23–29. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, G. Leaching of heavy metals from municipal solid waste incineration (MSWI) fly ash using sulfuric acid. Appl. Mech. Mater. 2013, 249, 922–926. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, G. Leaching of heavy metals from municipal solid waste incineration (MSWI) fly ash using nitric acid. Appl. Mech. Mater. 2013, 249, 918–921. [Google Scholar] [CrossRef]
- Chen, W.S.; Chang, F.C.; Shen, Y.H.; Tsai, M.S.; Ko, C.H. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012, 237, 116–120. [Google Scholar] [CrossRef]
- Xie, K.; Hu, H.; Cao, J.; Yang, F.; Liu, H.; Li, A.; Yao, H. A novel method for salts removal from municipal solid waste incineration fly ash through the molten salt thermal treatment. Chemosphere 2020, 241, 125107. [Google Scholar] [CrossRef]
- Zhu, F.; Takaoka, M.; Oshita, K.; Morisawa, S. The calcination process in a system for washing, calcinating, and converting treated municipal solid waste incinerator fly ash into raw material for the cement industry. J. Air Waste Manag. Assoc. 2011, 61, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Chimenos, J.M.; Fernández, A.I.; Cervantes, A.; Miralles, L.; Fernández, M.A.; Espiell, F. Optimizing the APC residue washing process to minimize the release of chloride and heavy metals. J. Waste Manag. 2005, 25, 686–693. [Google Scholar] [CrossRef]
- Drobíková, K.; Rozumová, L.; Otoupalíková, H.; Seidlerová, J. Bioleaching of hazardous waste. Chem. Papers 2015, 69, 1193–1201. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Asakura, Y.; Okada, N.; Koda, S.; Yasuda, K. Effect of ultrasonic cavitation on measurement of sound pressure using hydrophone. Jpn. J. Appl. Phys. 2017, 56, 07JE06. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Nguyen, T.T.; Asakura, Y. Measurement of distribution of broadband noise and sound pressures in sonochemical reactor. Ultrason. Sonochem. 2018, 43, 23–28. [Google Scholar] [CrossRef]
- Camargo-Perea, A.L.; Rubio-Clemente, A.; Peñuela, G.A. Use of ultrasound as an advanced oxidation process for the degradation of emerging pollutants in water. Water 2020, 12, 1068. [Google Scholar] [CrossRef] [Green Version]
- Naddeo, V.; Secondes, M.F.N.; Borea, L.; Hasan, S.W.; Ballesteros, F.; Belgiorno, V. Removal of contaminants of emerging concern from real wastewater by an innovative hybrid membrane process—UltraSound, Adsorption, and Membrane ultrafiltration (USAMe®). Ultrason. Sonochem. 2020, 68, 105237. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Liu, S.; Peng, Y.; Chen, J.; Ki, S.Y. Ultrasound-assisted electrochemical treatment for phenolic wastewater. Ultrason. Sonochem. 2020, 65, 105058. [Google Scholar] [CrossRef]
- Geng, N.; Chen, W.; Xu, H.; Ding, M.; Lin, T.; Wu, Q.; Zhang, L. Insights into the novel application of Fe-MOFs in ultrasound-assisted heterogeneous Fenton system: Efficiency, kinetics and mechanism. Ultrason. Sonochem. 2021, 72, 105411. [Google Scholar] [CrossRef]
- Yasuda, K. Sonochemical green technology using active bubbles: Degradation of organic substances in water. Curr. Opin. Green Sustain. Chem. 2021, 27, 100411. [Google Scholar] [CrossRef]
- Hasegawa, H.; Rahman, I.M.M.; Egawa, Y.; Sawai, H.; Begum, Z.A.; Maki, T.; Mizutani, S. Recovery of the rare metals from various waste ashes with the aid of temperature and ultrasound irradiation using chelants. Water Air Soil Pollut. 2014, 225, 13. [Google Scholar] [CrossRef] [Green Version]
- Biserčić, M.S.; Pezo, L.; Ignjatović, I.S.; Ignjatović, L.; Savić, A.; Jovanović, U.; Andric, V. Ultrasound and shacking-assisted water-leaching of anions and cations from fly ash. J. Serb. Chem. Soc. 2016, 81, 813–827. [Google Scholar] [CrossRef]
- Thakker, M.R.; Parikh, J.K.; Desai, M.A. Synergism between ionic liquid and ultrasound for greener extraction of geraniol: Optimization using different statistical tools, comparison and prediction. Chem. Eng. Res. Des. 2018, 134, 162–171. [Google Scholar] [CrossRef]
- Tsai, C.K.; Horng, J.J. Transformation of glass fiber waste into mesoporous zeolite-like nanomaterials with efficient adsorption of methylene blue. Sustainability 2021, 13, 6207. [Google Scholar] [CrossRef]
- Googerdchian, F.; Moheb, A.; Emadi, R.; Asgari, M. Optimization of Pb(II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J. Hazard. Mater. 2018, 349, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zolgharnein, J.; Rastgordani, M. Optimization of simultaneous removal of binary mixture of indigo carmine and methyl orange dyes by cobalt hydroxide nano-particles through Taguchi method. J. Mol. Liq. 2018, 262, 405–414. [Google Scholar] [CrossRef]
- Mustapha, A.N.; Zhang, Y.; Zhang, Z.; Ding, Y.; Yuan, Q.; Li, Y. Taguchi and ANOVA analysis for the optimization of the microencapsulation of a volatile phase change material. J. Mater. Res. Technol. 2021, 11, 667–680. [Google Scholar] [CrossRef]
- Gholami-Bonabi, L.; Ziaefar, N.; Sheikhloie, H. Removal of phenol from aqueous solutions by magnetic oxide graphene nanoparticles modified with ionic liquids using the Taguchi optimization approach. Water Sci. Technol. 2020, 81, 228–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Wang, M.; Wu, C.; Wang, X.; Yang, Y.; Liu, S.; Shimaoka, T.; Wang, Q. Dechlorination of fly ash by hydrolysate of municipal solid waste leachate. RSC Adv. 2020, 10, 26397–26406. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Zou, D.; Wu, C.; Li, T.; Gao, M.; Liu, S.; Wang, Q.; Shimaoka, T. Comparative study on inorganic Cl removal of municipal solid waste fly ash using different types and concentrations of organic acids. Chemosphere 2020, 261, 127754. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Asakura, Y.; Koda, S.; Yasuda, K. Dependence of cavitation, chemical effect, and mechanical effect thresholds on ultrasonic frequency. Ultrason. Sonochem. 2017, 39, 301–306. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Chen, H.H.; To, T.T.; Chang, Y.C.; Tsai, C.K.; Chen, K.F.; Tsai, Y.P. Development of biochars derived from water bamboo (Zizania latifolia) shoot husks using pyrolysis and ultrasound-assisted pyrolysis for the treatment of Reactive Black 5 (RB5) in wastewater. Water 2021, 13, 1615. [Google Scholar] [CrossRef]
- Huang, K.; Inoue, K.; Harada, H.; Kawakita, H.; Ohto, K. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans. Nonferrous Met. Soc. China 2011, 21, 1422–1427. [Google Scholar] [CrossRef]
- Mettin, R. Bubble structures in acoustic cavitation. In Bubble and Particle Dynamics in Acoustic Fields: Modern Trends and Applications; Doinikow, A.A., Ed.; Research Signpost: Trivandrum, India, 2005; pp. 1–37. [Google Scholar]
- Peter, A.; Chabot, B.; Loranger, E. Enhancing Surface Properties of Softwood Biochar by Ultrasound Assisted Slow Pyrolysis. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 2477–2480. [Google Scholar]
- Nguyen, T.T.; Takahashi, Y.; Asakura, Y.; Yasuda, K. Removal of silicic acid in geothermal water by a combination of ultrasonication and silica gel seed. J. Chem. Eng. Jpn. 2017, 50, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Ju, T.; Jiang, J.; Meng, Y.; Yan, F.; Xu, Y.; Gao, Y.; Aihemaiti, A. An investigation of the effect of ultrasonic waves on the efficiency of silicon extraction from coal fly ash. Ultrason. Sonochem. 2020, 60, 104765. [Google Scholar] [CrossRef]
- Asakura, Y.; Nishida, T.; Matsuoka, T.; Koda, S. Effects of ultrasonic frequency and liquid height on sonochemical efficiency of large-scale sonochemical reactors. Ultrason. Sonochem. 2008, 15, 244–250. [Google Scholar] [CrossRef]









| Physical Properties | Chemical Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture content (%) | pH | Surface area (m2/g) | Ca (%) | Cl (%) | Zn (%) | Fe (%) | Pb (%) | Cu (%) | Ba (%) | Cr (%) | Cd (%) | Ni (%) |
| 4.02 | 12.02 | 13.05 | 24.72 | 32.15 | 0.87 | 0.27 | 0.21 | 0.075 | 0.009 | 0.011 | 0.015 | 0.007 |
| Delta | ||||
|---|---|---|---|---|
| A | B | C | D | |
| Citric | 1.599 | 6.498 | 22.940 | 3.144 |
| Ascorbic | 2.239 | 3.402 | 19.585 | 2.625 |
| EDTA-Na2 | 2.724 | 2.630 | 13.561 | 0.501 |
| Wt% | ||||
|---|---|---|---|---|
| O | Ca | Cl | C | |
| Raw fly ash | 32.8 | 31.7 | 21 | 13.9 |
| Citric | 49.7 | 15.6 | 0.3 | 26 |
| Ascorbic | 31.8 | 10.3 | 0.3 | 44.7 |
| EDTA-Na2 | 49.5 | 20.3 | 0.3 | 22.6 |
| Surface Area (m2/g) | |
|---|---|
| Raw fly ash | 13.05 |
| EDTA-Na2 at 165 kHz, 250 W, 5 min | 91.47 |
| EDTA-Na2 at 165 kHz, 250 W, 20 min | 27.02 |
| EDTA-Na2 at 20 kHz, 250 W, 5 min | 98.06 |
| EDTA-Na2 at 20 kHz, 250 W, 20 min | 27.59 |
| Symbol | Factors | Unit | Levels | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| A | Conc. | mol L−1 | Citric | 0.1 | 0.25 | 0.5 | 0.75 |
| Ascorbic | 0.1 | 0.25 | 0.5 | 0.75 | |||
| EDTA-Na2 | 0.01 | 0.05 | 0.1 | 0.25 | |||
| B | Time | min | 5 | 10 | 15 | 20 | |
| C | S/L ratio | - | 1:2.5 | 1:5 | 1:7.5 | 1:10 | |
| D | Position | mm | 5 | 20 | 30 | 40 | |
| Exp. No. | Factors | |||
|---|---|---|---|---|
| A | B | C | D | |
| 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 | 2 |
| 3 | 1 | 3 | 3 | 3 |
| 4 | 1 | 4 | 4 | 4 |
| 5 | 2 | 1 | 2 | 3 |
| 6 | 2 | 2 | 1 | 4 |
| 7 | 2 | 3 | 4 | 1 |
| 8 | 2 | 4 | 3 | 2 |
| 9 | 3 | 1 | 3 | 4 |
| 10 | 3 | 2 | 4 | 3 |
| 11 | 3 | 3 | 1 | 2 |
| 12 | 3 | 4 | 2 | 1 |
| 13 | 4 | 1 | 4 | 2 |
| 14 | 4 | 2 | 3 | 1 |
| 15 | 4 | 3 | 2 | 4 |
| 16 | 4 | 4 | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Tsai, C.-K.; Horng, J.-J. Sustainable Recovery of Valuable Nanoporous Materials from High-Chlorine MSWI Fly Ash by Ultrasound with Organic Acids. Molecules 2022, 27, 2289. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072289
Nguyen TT, Tsai C-K, Horng J-J. Sustainable Recovery of Valuable Nanoporous Materials from High-Chlorine MSWI Fly Ash by Ultrasound with Organic Acids. Molecules. 2022; 27(7):2289. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072289
Chicago/Turabian StyleNguyen, Tam Thanh, Cheng-Kuo Tsai, and Jao-Jia Horng. 2022. "Sustainable Recovery of Valuable Nanoporous Materials from High-Chlorine MSWI Fly Ash by Ultrasound with Organic Acids" Molecules 27, no. 7: 2289. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27072289