Removal of Copper(II) in the Presence of Sodium Dodecylobenzene Sulfonate from Acidic Effluents Using Adsorption on Ion Exchangers and Micellar-Enhanced Ultrafiltration Methods
Abstract
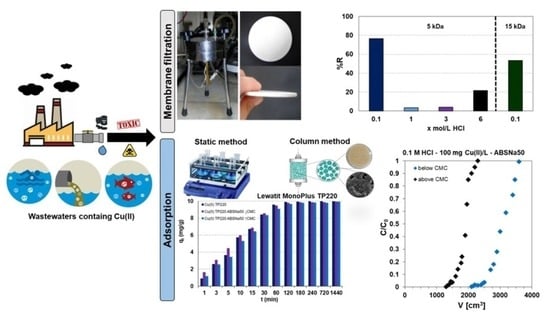
:1. Introduction
2. Results
2.1. Characterization of Adsorbents
2.2. Kinetic Studies of Cu(II) Adsorption
- –
- sorption capacity (qe) (mg/g):
- –
- the amount of Cu(II) ions adsorbed after time t (qt) per weight unit of sorbent under non-equilibrium conditions:
- –
- percentage removal (%R):where C0 (mg/L)—the initial concentration of Cu(II) in the water phase, Ce (mg/L)—the equilibrium concentrations of Cu(II) in the water phase, Ct (mg/L)—the concentration of Cu(II) in the water phase after t, V (L)—the volume of solution, and m (g)—the mass of dry sorbent.
2.3. Kinetic and Isotherm Parameters of Adsorption System
2.3.1. Kinetic Parameters of Adsorption
- PFO: non-linear equation (NL), linear equation (L), kinetic parameters (KP)
- PSO:
- IPD:
- Error analysis:
- -
- MPSD—the Marquardt’s percent standard deviation:
- -
- R2—the determination coefficient:
- -
- the adjusted R-squaredwhere qe and qt (mg/g)—the Cu(II) amounts sorbed at the equilibrium and at time t; k1 (1/min), k2 (g/mg min), ki (mg/g min0.5)—the rate constants of Cu(II) adsorption determined from the PFO, PSO, and IPD models; h (mg/g min)—the initial sorption rate; qe,exp (mg/g)—the amount of Cu(II) adsorbed at equilibrium; qe,cal (mg/g)—the calculated amount of Cu(II) adsorbed; qe,mean (mg/g)—measured by the means of the qe,exp values; k—the number of independent regressors; and n—the points number.
2.3.2. Isotherm Parameters of Adsorption
2.4. Column Studies
- –
- the weight distribution coefficient (Dw):where U″(mL)—the eluate volume for C/C0 = 0.5, U0 (mL) —the dead column volume (U0 = 2 mL), V (mL)—the free volume (intergranular) of the ion exchange bed (about 0.4 bed volume), and mj (g)—the mass of dry ion exchange resin in the column;
- –
- the bed distribution coefficient (Db):where dz (g/mL)—the ion exchange density (determined experimentally by drying the appropriate amount of ion exchanger to the constant mass). The working ion exchange capacity (Cw) (g/mL):where Up (L)—the volume of eluate to break through the column, C0 (g/L)—initial concentration of Cu(II) in the solution, and Vj (mL)—the volume of the ion exchange in the column.
2.5. Lewatit MonoPlus TP220 Characterization after the Cu(II) Adsorption in the Presence of ABSNa50 Surfactant
2.6. Copper(II) Removal by the Micellar-Enhanced Ultrafiltration (MEUF)
2.7. General Remarks
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Physical Characterization of Adsorbents
3.3. Sorption Studies by the Static and Column Tests
3.4. Modelling of Kinetic and Equilibrium Studies of Cu(II) Sorption
3.5. Copper(II) Removal by the Micellar-Enhanced Ultrafiltration (MEUF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Heavy Metal Removal by Fixed-Bed Column—A Review. ChemBioEng Rev. 2018, 5, 173–179. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ojstršek, A.; Vouk, P.; Fakin, D. Adsorption of Pollutants from Colored Wastewaters after Natural Wool Dyeing. Materials 2022, 15, 1488. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Ferella, F.; Prisciandaro, M.; De Michelis, I.; Veglio’, F. Removal of Heavy Metals by Surfactant-Enhanced Ultrafiltration from Wastewaters. Desalination 2007, 207, 125–133. [Google Scholar] [CrossRef]
- Jancevičiūtė, R.; Gefenienė, A. Sorption of Copper (Ii) and Nonionic Surfactant by Ion Exchangers and Activated Carbon. J. Environ. Eng. Landsc. Manag. 2006, 14, 191–197. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A Review on Adsorbents for Treatment of Water and Wastewaters Containing Copper Ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Removal of Surfactant from Industrial Wastewaters by Coagulation Flocculation Process. J. Environ. Sci. Technol. 2006, 3, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.K.; Li, C.W.; Lin, C.Y. Micellar-Enhanced Ultrafiltration Process (MEUF) for Removing Copper from Synthetic Wastewater Containing Ligands. Chemosphere 2004, 57, 629–634. [Google Scholar] [CrossRef]
- Petrov, S.; Nenov, V. Removal and Recovery of Copper from Wastewater by a Complexation-Ultrafiltration Process. Desalination 2004, 162, 201–209. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schmidt, E. Polymer-Enhanced Ultrafiltration Process for Heavy Metals Removal from Industrial Wastewater. Desalination 2010, 256, 90–93. [Google Scholar] [CrossRef]
- Juang, R.S.; Xu, Y.Y.; Chen, C.L. Separation and Removal of Metal Ions from Dilute Solutions Using Micellar-Enhanced Ultrafiltration. J. Memb. Sci. 2003, 218, 257–267. [Google Scholar] [CrossRef]
- Huang, Y.C.; Batchelor, B.; Koseoglu, S.S. Crossflow Surfactant-Based Ultrafiltration of Heavy Metals from Waste Streams. Sep. Sci. Technol. 1994, 29, 1979–1998. [Google Scholar] [CrossRef]
- Huang, Y.C.; Batchelor, B.; Koseoglu, S.S. Surfactant-Enhanced Ultrafiltration of Heavy Metals from Waste Streams with Pilot-Scale System. Hazard. Waste Hazard. Mater. 1994, 11, 385–395. [Google Scholar] [CrossRef]
- Ahmadi, S.; Huang, Y.-C.; Batchelor, B.; Koseoglu, S.S. Binding of Heavy Metals to Derivatives of Cholesterol and Sodium Dodecyl Sulfate. J. Environ. Eng. 1995, 121, 645–652. [Google Scholar] [CrossRef]
- Tondre, C.; Son, S.G.; Hebrant, M.; Scrimin, P.; Tecilla, P. Micellar Extraction: Removal of Copper(II) by Micelle-Solubilized Complexing Agents of Varying HLB Using Ultrafiltration. Langmuir 1993, 9, 950–955. [Google Scholar] [CrossRef]
- Scamehorn, J.F.; Christian, S.D.; El-Sayed, D.A.; Uchiyama, H.; Younis, S.S. Removal of Divalent Metal Cations and Their Mixtures from Aqueous Streams Using Micellar-Enhanced Ultrafiltration. Sep. Sci. Technol. 1994, 29, 809–830. [Google Scholar] [CrossRef]
- Liu, C.K.; Li, C.W. Recovery of Copper and DCA from Simulated Micellar Enhanced Ultrafiltration (MEUF) Waste Stream. World Acad. Sci. Eng. Technol. 2009, 59, 92–97. [Google Scholar] [CrossRef]
- Arbabi, M.; Golshani, N. Removal of Copper Ions Cu (II) from Industrial Wastewater: A Review of Removal Methods. Int. J. Epidemiol. Res. 2016, 3, 283–293. [Google Scholar]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper Removal from Industrial Wastewater: A Comprehensive Review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. The Use of the Chelating Resin of a New Generation Lewatit MonoPlus TP-220 with the Bis-Picolylamine Functional Groups in the Removal of Selected Metal Ions from Acidic Solutions. Chem. Eng. J. 2012, 197, 493–508. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Sofińska-Chmiel, W.; Mendyk, E.; Hubicki, Z. DOWEX M 4195 and LEWATIT® MonoPlus TP 220 in Heavy Metal Ions Removal from Acidic Streams. Sep. Sci. Technol. 2014, 49, 2003–2015. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Ion Exchange Recovery of Palladium(II) from Acidic Solutions Using Monodisperse LewatitSR-7. Ind. Eng. Chem. Res. 2012, 51, 16688–16696. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Applicability of New Acrylic, Weakly Basic Anion Exchanger Purolite A-830 of Very High Capacity in Removal of Palladium(II) Chloro-Complexes. Ind. Eng. Chem. Res. 2012, 51, 7223–7230. [Google Scholar] [CrossRef]
- Kaneko, K. Determination of Pore Size and Pore Size Distribution: 1. Adsorbents and Catalysts. J. Memb. Sci. 1994, 96, 59–89. [Google Scholar] [CrossRef]
- De Lange, M.F.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Adsorptive Characterization of Porous Solids: Error Analysis Guides the Way. Microporous Mesoporous Mater. 2014, 200, 199–215. [Google Scholar] [CrossRef]
- Water Treatment and Purification-Lenntech. Available online: https://www.lenntech.com/ (accessed on 5 April 2022).
- Araucz, K.; Aurich, A.; Kołodyńska, D. Novel Multifunctional Ion Exchangers for Metal Ions Removal in the Presence of Citric Acid. Chemosphere 2020, 251, 126331. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Enhanced Removal of Copper(II) from Acidic Streams Using Functional Resins: Batch and Column Studies. J. Mater. Sci. 2020, 55, 13687–13715. [Google Scholar] [CrossRef]
- Balistrieri, L.S.; Murray, J.W. The Surface Chemistry of Goethite (Alpha FeOOH) in Major Ion Seawater. Am. J. Sci. 1981, 281, 788–806. [Google Scholar] [CrossRef]
- Wołowicz, A.; Staszak, K.; Hubicki, Z. Static Sorption of Heavy Metal Ions on Ion Exchanger in the Presence of Sodium Dodecylbenzenesulfonate. Adsorption 2019, 25, 393–404. [Google Scholar] [CrossRef] [Green Version]
- King, P.; Srinivas, P.; Kumar, Y.P.; Prasad, V.S.R.K. Sorption of Copper(II) Ion from Aqueous Solution by Tectona Grandis l.f. (Teak Leaves Powder). J. Hazard. Mater. 2006, 136, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Harland, C.E. Ion Exchange: Theory and Practice; RSC Paperbacks; Royal Society of Chemistry: Cambridge, UK, 1994; ISBN 978-0-85186-484-6. [Google Scholar]
- Pan, N.; Pietrzyk, D.J. Separation of Anionic Surfactants on Anion Exchangers. J. Chromatogr. A 1995, 706, 327–337. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Low, A.; Rabat, N.E. A Review on Recent Developments in the Adsorption of Surfactants from Wastewater. J. Environ. Manag. 2020, 254, 109797. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, J.; Boika, A.; Bard, A.J. Electrochemistry of High Concentration Copper Chloride Complexes. Anal. Chem. 2013, 85, 7696–7703. [Google Scholar] [CrossRef]
- Kononova, O.N.; Karplyakova, N.S.; Duba, E.V. Sorption Recovery of Platinum(II,IV) in Presence of Copper(II) and Zinc(II) from Chloride Solutions. J. Serb. Chem. Soc. 2015, 80, 1149–1160. [Google Scholar] [CrossRef]
- Kononova, O.N.; Kuznetsova, M.A.; Melnikov, A.M.; Karplyakova, N.S.; Kononov, Y.S. Sorption Recovery of Copper(II) and Zinc(II) from Aqueous Chloride Solutions. J. Serb. Chem. Soc. 2014, 79, 1037–1049. [Google Scholar] [CrossRef]
- Rahmani, S.; Mohseni, M. The Role of Hydrophobic Properties in Ion Exchange Removal of Organic Compounds from Water. Can. J. Chem. Eng. 2017, 95, 1449–1455. [Google Scholar] [CrossRef]
- Croué, J.-P.; Violleau, D.; Bodaire, C.; Legube, B. Removal of Hydrophobic and Hydrophilic Constituents by Anion Exchange Resin. Water Sci. Technol. 1999, 40, 207–214. [Google Scholar] [CrossRef]
- Li, P.; SenGupta, A.K. Sorption of Hydrophobic Ionizable Organic Compounds (HIOCs) onto Polymeric Ion Exchangers. React. Funct. Polym. 2004, 60, 27–39. [Google Scholar] [CrossRef]
- Li, P.; SenGupta, A.K. Entropy-Driven Selective Ion Exchange for Aromatic Ions and the Role of Cosolvents. Colloids Surf. A Physicochem. Eng. Asp. 2001, 191, 123–132. [Google Scholar] [CrossRef]
- Glerup, J.; Goodson, P.A.; Hodgson, D.J.; Michelsen, K.; Nielsen, K.M.; Weihe, H. Synthesis and Characterization of Bis(2-Pyridylmethyl)Amine Complexes of Manganese(II), Zinc(II), and Cadmium(II). Inorg. Chem. 2002, 31, 4611–4616. [Google Scholar] [CrossRef]
- Zhu, Y.; Millan, E.; Sengupta, A.K. Toward Separation of Toxic Metal(II) Cations by Chelating Polymers: Some Noteworthy Observations. React. Polym. 1990, 13, 241–253. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; SenGupta, A.K. Interference-Free Detection of Trace Copper in the Presence of EDTA and Other Metals Using Two Complementary Chelating Polymers. Colloids Surf. A Physicochem. Eng. Asp. 2011, 1–3, 432–441. [Google Scholar] [CrossRef]
- Grinstead, R.R. New Developments in the Chemistry of XFS 4195 and XFS 43084 Chelating Ion Exchange Resins. In Proceedings of the Ion Exchange Technology; Naden, D., Streat, M., Eds.; Horwood: Chichester, UK, 1984; pp. 509–518. [Google Scholar]
- Sengupta, A.K.; Zhu, Y.; Hauze, D. Metal(II) Ion Binding onto Chelating Exchangers with Nitrogen Donor Atoms: Some New Observations and Related Implications. Environ. Sci. Technol. 1991, 25, 481–488. [Google Scholar] [CrossRef]
- Diniz, C.V.; Doyle, F.M.; Ciminelli, V.S.T. Effect of PH on the Adsorption of Selcted Heavy Metal Ions Form Concentrated Chloride Solutions by the Chelating Resin Dowex M-4195. Sep. Sci. Technol. 2006, 37, 3169–3185. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial Behavior of Intraparticle Diffusion Model Used in the Description of Adsorption Kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Kalavathy, M.H.; Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Kinetic and Isotherm Studies of Cu(II) Adsorption onto H3PO4-Activated Rubber Wood Sawdust. J. Colloid Interface Sci. 2005, 292, 354–362. [Google Scholar] [CrossRef]
- Sofińska-Chmiel, W.; Kołodyńska, D.; Adamczuk, A.; Świetlicki, A.; Goliszek, M.; Smagieł, R. Studies on the Mechanism of Cu(II) Ion Sorption on Purolite S 940 and Purolite S 950. Materials 2021, 14, 2915. [Google Scholar] [CrossRef]
- Bulai, P.; Balan, C.; Scripcariu, C.; Macoveanu, M. Equlibrium and Kinetic Studies of Copper(II) Removal on Purolite S930 Resins. Environ. Eng. Manag. 2009, 8, 1103–1109. [Google Scholar] [CrossRef]
- Snukiškis, J.; Kaušpédiené, D. Kinetics of the Smultaneous Sorption Ofnonionic Surfactant and Copper(II) by Poly Acrylic Acid- Functionalized Ion Exchanher. Solvent Extr. Ion Exch. 2000, 18, 1001–1013. [Google Scholar] [CrossRef]
- Kaušpediene, D.; Snukiškis, J.; Gefeniene, A. Kinetic Behaviour of Duolite ES 468 in the Cosorption of Non-Ionic Surfactant and Copper(II). J. Hazard. Mater. 2003, 99, 313–319. [Google Scholar] [CrossRef]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic Modelling of the Adsorption of Nitrates by Ion Exchange Resin. Chem. Eng. J. 2006, 125, 111–117. [Google Scholar] [CrossRef]
- Liu, C.; Ngo, H.H.; Guo, W. Watermelon Rind: Agro-Waste or Superior Biosorbent? Appl. Biochem. Biotechnol. 2012, 167, 1699–1715. [Google Scholar] [CrossRef]
- Gupta, H.; Gogate, P.R. Intensified Removal of Copper from Waste Water Using Activated Watermelon Based Biosorbent in the Presence of Ultrasound. Ultrason. Sonochem. 2016, 30, 113–122. [Google Scholar] [CrossRef]
- Imamoglu, M.; Tekir, O. Removal of Copper (II) and Lead (II) Ions from Aqueous Solutions by Adsorption on Activated Carbon from a New Precursor Hazelnut Husks. Desalination 2008, 1–3, 108–113. [Google Scholar] [CrossRef]
- Snukiškis, J.; Kaušpediene, D. Kinetics of the Combined Sorption of Copper(II) and Nonionic Surfactant by Carboxylic Acrylcationic Exchanger. Sep. Purif. Technol. 2001, 24, 59–65. [Google Scholar] [CrossRef]
- Minczewski, J. Separation and Preconcentration Methods in Inorganic Trace Analysis; Horwood, E., Ed.; Halsted Press: Chichester West Sussex, UK, 1982; ISBN 9780853121657. [Google Scholar]
- Lin, W.; Jing, L.; Zhu, Z.; Cai, Q.; Zhang, B. Removal of Heavy Metals from Mining Wastewater by Micellar-Enhanced Ultrafiltration (MEUF): Experimental Investigation and Monte Carlo-Based Artificial Neural Network Modeling. Water Air Soil Pollut. 2017, 228, 206. [Google Scholar] [CrossRef]
- Sharma, P.; Sarkar, B. Prediction of Permeate Flux during Ultrafiltration of Polysaccharide in a Stirred Batch Cell. Food Bioprocess. Technol. 2012, 6, 3634–3643. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of PH on Heavy Metal Speciation and Removal from Wastewater Using Micellar-Enhanced Ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chem. Senses 2006, 31, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.; Mazur, L.P.; Weschenfelder, S.E.; Regis, R.J.; de Souza, R.A.F.; Marinho, B.A.; da Silva, A.; de Souza, S.M.A.G.U.; de Souza, A.A.U. Water and Wastewater Treatment by Micellar Enhanced Ultrafiltration—A Critical Review. J. Water Process. Eng. 2022, 46, 102574. [Google Scholar] [CrossRef]
- Xu, K.; Zeng, G.M.; Huang, J.H.; Wu, J.Y.; Fang, Y.Y.; Huang, G.; Li, J.; Xi, B.; Liu, H. Removal of Cd2+ from Synthetic Wastewater Using Micellar-Enhanced Ultrafiltration with Hollow Fiber Membrane. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 140–146. [Google Scholar] [CrossRef]
- Tortora, F.; Innocenzi, V.; De Michelis, I.; Vegliò, F.; Di Celso, G.M.; Prisciandaro, M. Recovery of Anionic Surfactant Through Acidification/Ultrafiltration in a Micellar-Enhanced Ultrafiltration Process for Cobalt Removal. Environ. Eng. Sci. 2018, 35, 493–500. [Google Scholar] [CrossRef]
- Huisman, I.H.; Trägårdh, G.; Trägårdh, C.; Pihlajamäki, A. Determining the Zeta-Potential of Ceramic Microfiltration Membranes Using the Electroviscous Effect. J. Memb. Sci. 1998, 147, 187–194. [Google Scholar] [CrossRef]
- Tung, C.C.; Yang, Y.M.; Chang, C.H.; Maa, J.R. Removal of Copper Ions and Dissolved Phenol from Water Using Micellar-Enhanced Ultrafiltration with Mixed Surfactants. Waste Manag. 2002, 22, 695–701. [Google Scholar] [CrossRef]
- Li, C.W.; Liu, C.K.; Yen, W.S. Micellar-Enhanced Ultrafiltration (MEUF) with Mixed Surfactants for Removing Cu(II) Ions. Chemosphere 2006, 63, 353–358. [Google Scholar] [CrossRef]
- Bade, R.; Lee, S.H. Micellar Enhanced Ultrafiltration and Activated Carbon Fibre Hybrid Processes for Copper Removal from Wastewater. Korean J. Chem. Eng. 2007, 24, 239–245. [Google Scholar] [CrossRef]
- Schwarze, M.; Chiappisi, L.; Prévost, S.; Gradzielski, M. Oleylethoxycarboxylate—An Efficient Surfactant for Copper Extraction and Surfactant Recycling via Micellar Enhanced Ultrafiltration. J. Colloid Interface Sci. 2014, 421, 184–190. [Google Scholar] [CrossRef]
- Kowalska, I.; Klimonda, A. Removal of Copper Ions from Aqueous Solutions by Means of Micellar-Enhanced Ultrafiltration. In Proceedings of the E3S Web of Conferences, International Conference on Advances in Energy Systems and Environmental Engineering (ASEE17), Wrocław, Poland, 2–5 July 2017; Volume 22, p. 00087. [Google Scholar]
- Sarkar, B. Micellar enhanced ultrafiltration in the treatment of dye wastewater: Fundamentals, state-of-the-art and future perspectives. Groundw. Sustain. Dev. 2022, 17, 100730. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Fruendlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physicochim. URSS 1940, 12, 217–222. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]















| Sorbent | Type | Matrix | Structure | Functional Groups | Mean Bead Size (mm) | Total Capacity (val/L) | Water Retention (%) |
|---|---|---|---|---|---|---|---|
| AF5 | AWFG | Carbonaceous | Micr. | without | 0.4–0.8 | – | 48–60 |
| S984 | CHIE | Cross-linked polyacrylic | Macr. | Polyamine | – | 2.7 | 44–55 |
| TP220 | CHIE | Cross-linked polystyrene | Macr. | Bis-picolylamine, bis(2-pyridyl-methyl)amine | 0.62 (±0.05) | 2.2 | 48–60 |
| A830 | WBA | Cross-linked polyacrylic | Macr. | Complex amine | 0.3–1.2 | 2.75 | 47–53 |
| A400TL | SBA | Cross-linked polyacrylic | Micr. | Quaternary ammonium, type 1 | 0.425–0.85 | 1.3 | 48–54 |
| SR7 | SBA | Cross-linked polystyrene | Macr. | Quaternary ammonium, type 3 | 0.57–0.67 | 0.6 | 59–64 |
| PSR2 | SBA | Cross-linked polystyrene | Micr. | Quaternary ammonium, tri-n-butyl amine type | 0.3–1.2 | 0.65 | 40–48 |
| PSR3 | SBA | Cross-linked polystyrene | Macr. | 0.3–1.2 | 0.6 | 50–65 |
| Sorbent | %N | %C | %H | %S | SBET (m2/g) | Ps (nm) | Pv (cm3/g) |
|---|---|---|---|---|---|---|---|
| TP220 | 9.26 | 70.58 | 7.81 | 0.24 | 21.38 | 48.20 | 0.258 |
| SR7 | 2.58 | 70.79 | 6.49 | 0.26 | 19.69 | 44.61 | 0.220 |
| AF5 | 0.02 | 93.81 | 1.58 | 0.91 | 988.77 | 2.31 | 0.572 |
| A400TL | 4.54 | 59.64 | 9.76 | 0.24 | 4.20 | 2.35 | 0.002 |
| A830 | 16.35 | 46.00 | 8.39 | 0.00 | 9.66 | 48.83 | 0.012 |
| S984 | 18.13 | 46.95 | 7.62 | 0.87 | 3.80 | 89.36 | 0.085 |
| PSR2 | 2.49 | 71.38 | 8.02 | 1.28 | 6.32 | 10.08 | 0.016 |
| PSR3 | 2.25 | 73.89 | 10.89 | 0.06 | 6.36 | 3.65 | 0.006 |
| Parameters | HCl−Cu(II)−ABSNa50 | ||
|---|---|---|---|
| ABSNa50 ↑CMC | ABSNa50 ↓CMC | ||
| qe,exp (mg/g) | 9.97 | 9.99 | |
| PFO—L | qe,cal (mg/g) | 2.39 | 1.08 |
| k1 (1/min) | 0.006 | 0.003 | |
| R2 | 0.476 | 0.277 | |
| PFO—NL | qe,cal (mg/g) | 9.25 | 9.63 |
| k1 (1/min) | 0.103 | 0.096 | |
| R2 | 0.975 | 0.989 | |
| R2adj | 0.969 | 0.986 | |
| MPSD | 0.115 | 0.026 | |
| PSO—L | qe,cal (mg/g) | 10.00 | 10.01 |
| k2 (g/mg min) | 0.014 | 0.019 | |
| R2 | 1.000 | 1.000 | |
| h (mg/g min) | 1.44 | 1.89 | |
| PSO—NL | qe,cal (mg/g) | 10.16 | 10.61 |
| k2 (g/mg min) | 0.012 | 0.010 | |
| MPSD | 0.027 | 0.027 | |
| R2 | 0.996 | 0.991 | |
| R2adj | 0.995 | 0.989 | |
| IPD | qe,cal (mg/g) | 14.47 | 14.57 |
| ki (mg/g min0.5) | 0.18 | 0.18 | |
| R2 | 0.924 | 0.757 | |
| R2adj | 0.773 | 0.270 | |
| Model | Parameters | HCl–Cu(II)–ABSNa50 | |
|---|---|---|---|
| ABSNa50 ↑CMC | ABSNa50 ↓CMC | ||
| qe,exp (mg/g) | 130.38 | 110.00 | |
| Linear regression | |||
| Langmuir | Q0 (mg/g) | 129.54 | 109.08 |
| kL (L/mg) | 0.021 | 0.044 | |
| R2 | 0.998 | 1.000 | |
| Freundlich | kF (mg1−1/n L1/n/g) | 19.20 | 21.04 |
| 1/n | 0.249 | 0.221 | |
| R2 | 0.718 | 0.711 | |
| Temkin | bT (J g/mol mg) | 204.49 | 258.65 |
| A (L/mg) | 10.494 | 32.460 | |
| R2 | 0.922 | 0.890 | |
| Dubinin–Radushkevich | qm (mg/g) | 92.82 | 83.14 |
| kDR (mol2 J2) | 3.6 × 10−7 | 1.6 × 10−7 | |
| E (kJ/mol) | 1.177 | 1.787 | |
| R2 | 0.911 | 0.901 | |
| Non-linear regression | |||
| Langmuir | Q0 (mg/g) | 124.06 | 108.47 |
| kL (L/mg) | 0.137 | 0.298 | |
| MPSD | 0.523 | 0.285 | |
| R2 | 0.962 | 0.978 | |
| 0.951 | 0.972 | ||
| Freundlich | kF (mg1−1/n L1/n/g) | 9.89 | 19.01 |
| 1/n | 0.313 | 0.224 | |
| MPSD | 2.244 | 4.149 | |
| R2 | 0.790 | 0.774 | |
| 0.731 | 0.709 | ||
| Temkin | bT (J g/mol mg) | 165.65 | 208.77 |
| A (L/mg) | 3.297 | 7.570 | |
| MPSD | 0.488 | 0.534 | |
| R2 | 0.922 | 0.890 | |
| 0.899 | 0.859 | ||
| Dubinin–Radushkevich | qm (mg/g) | 150.61 | 64.48 |
| kDR (mol2 J2) | 9 × 10−7 | 1.5 × 10−7 | |
| E (J/mol) | 745.54 | 1827.56 | |
| MPSD | 2.910 | 1.488 | |
| R2 | 0.953 | 0.851 | |
| 0.940 | 0.809 | ||
| Adsorbate/Adsorbent | Conditions | Isotherm Models Sorption Capacity | Ref. | |
|---|---|---|---|---|
| Cu(II) | H3PO4-activated rubber wood sawdust SBET = 1673.86 m2/g | 0.5 g; 100 mL; pH 6; initial; 20 mg/L | Langmuir, qmax = 5.73 mg/g (303 K) qmax = 5.70 mg/g (308 K) qmax = 5.49 mg/g (313 K) | [52] |
| Cu(II) | watermelon rind | 0.5 g; pH 5; 10 mg/L; 20 °C | Langmuir, qmax = 6.28 mg/g | [58] |
| Cu(II) | 1 g; pH 5; 10 mg/L; 40 °C | Langmuir, qmax = 31.25 mg/g | [59] | |
| Cu(II) | AC from hazelnut husks * SBET = 1092 m2/g | 0.05–0.5 g; 25 mL; 200 rpm | Langmuir, qmax = 6.65 mg/g | [60] |
| Ion exchangers | ||||
| Cu(II) | Lewatit MonoPlus TP220 styrene divinylbenzene FG: bis-picolylamine | 0.5 g; V = 50 mL; 100–10000 mg/L; 180 rpm; t = 24 h | Langmuir, qmax = 230.2 mg/g | [30] |
| Cu(II) | Purolite S940, Purolite S950 styrene divinylbenzene FG: aminophosphonic | 0.20 g; 20 mL of 0.001 mol/L CuCl2; 180 rpm; T = 298 K; T = 2 h | Based on the kinetic studies: q = 8.17 mg/g, S940 q = 9.72 mg/g, S950 | [53] |
| Cu(II) | Purolite S930, ST-DVB FG: iminodiacetic acid | 1 g/L; pH 3; 100, 300 mg/L; T = 293, 303 K; t = 1 min–24 h | Langmuir, qmax = 133.33 mg/g | [54] |
| OS-20 + Cu(II) + H2O | Purolite C106 polyacrylic, FG: carboxyl groups | 0.5 g; 100 mL; pH 3–5; 6 mmol/L; 20 °C | Based on the kinetic studies: q = 0.22 mmol/g, pH 3 q = 0.21 mmol/g, pH 5 | [55] |
| ALM-10 + Cu(II) + H2O | Duolite ES 468 polyacrylic, FG: carboxyl groups | 0.5 g; 100 mL; pH 3–5; 6 mmol/L; 20 °C | Based on the kinetic studies: q = 1.07 mmol/g, pH 3 q = 1.39 mmol/g, pH 5 | [56] |
| ALM-10 + Cu(II) + H2O | Purolite C106 polyacrylic, FG: carboxyl groups | 0.5 g; 100 mL; pH 3–5; 6 mmol/L; 20 °C | Based on the kinetic studies: q = 0.61 mmol/kg, pH 3 q = 0.46 mmol/kg, pH 5 | [61] |
| Cu(II) + HCl + ABSNa50 ↓CMC | Lewatit MonoPlus TP220 styrene divinylbenzene FG: bis-picolylamine | 0.5 g; V = 50 mL; 50–8000 mg/L; 180 rpm; t = 24 h | Langmuir, qmax = 130.38 mg/g | This paper |
| Cu(II) + HCl + ABSNa50 ↑CMC | 0.5 g; V = 50 mL; 50–8000 mg/L; 180 rpm, t = 24 h | Langmuir, qmax = 110 mg/g | This paper | |
| Surfactant | Membrane | Pore Size | %R | Ref. |
|---|---|---|---|---|
| SDS/Triton X-100 10 mmol/L | cellulose, Sa = 60 cm2 | 5 kDa | 85% | [71] |
| SDS/Triton X-100 5.67/1.29 mmol/L | cellulose, YM10 | 10 kDa | 92% | [72] |
| Brij 35:SDS 0.3 TW-80:SDS 0.3 Triton X-100:SDS 0.7 | Polysulfone | 6 kDa | 98.3% 95.8% 93.5% | [37] |
| SDS | polyacrylonitrile Sa = 550 cm2 | 5 kDa | 98% | [73] |
| SDS/ligands * | Amicon hydrophilic YM10 membrane | 10 kDa | >95% (without ligands); <40% (with ligands); <10% (SDS > 25 mmol/L) | [10] |
| CPC/ligands * | 100% (EDTA: Cu = 1); 50% (EDTA: Cu = 24); 90% (NTA) | |||
| RO90 | cellulose PLCC | 5 kDa | 98% | [74] |
| SDBS | polysulfone membranes, Sa ≈ 900 cm2 | 5 kDa | >90% | [75] |
| ABSNa50ABSNa50 (5CMC) | ceramic membrane ** Sa ≈ 64 cm2 | 5 kDa | 76.46% (0.1 mol/L HCl) | This paper |
| Isotherm | Non-Linear Forms | Equation Number | Linear Forms | Equation Number |
|---|---|---|---|---|
| Langmuir | (23) | (24) | ||
| Freundlich | (25) | (26) | ||
| Temkin | (27) | (28) | ||
| Dubinin– Radushkevich | (29) | (32) | ||
| (30) | ||||
| (31) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołowicz, A.; Staszak, K.; Hubicki, Z. Removal of Copper(II) in the Presence of Sodium Dodecylobenzene Sulfonate from Acidic Effluents Using Adsorption on Ion Exchangers and Micellar-Enhanced Ultrafiltration Methods. Molecules 2022, 27, 2430. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082430
Wołowicz A, Staszak K, Hubicki Z. Removal of Copper(II) in the Presence of Sodium Dodecylobenzene Sulfonate from Acidic Effluents Using Adsorption on Ion Exchangers and Micellar-Enhanced Ultrafiltration Methods. Molecules. 2022; 27(8):2430. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082430
Chicago/Turabian StyleWołowicz, Anna, Katarzyna Staszak, and Zbigniew Hubicki. 2022. "Removal of Copper(II) in the Presence of Sodium Dodecylobenzene Sulfonate from Acidic Effluents Using Adsorption on Ion Exchangers and Micellar-Enhanced Ultrafiltration Methods" Molecules 27, no. 8: 2430. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules27082430