Novel Synthesis of IMC-48 and Affinity Evaluation with Different i-Motif DNA Sequences
Abstract
:1. Introduction
2. Results and Discussion
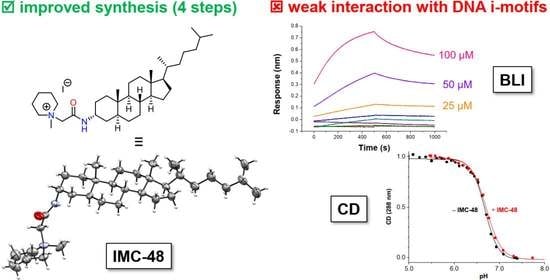
2.1. Novel Synthesis of IMC-48
2.2. Evaluation by BLI of the Affinity of IMC-48 for DNA Sequences
2.3. Circular Dichroism Studies
3. Conclusions
4. Experimental Section
4.1. Crystal Structure Analysis
4.2. Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References and Note
- Varshney, D.; Spiegel, J.; Zyner, K.; Tannahill, D.; Balasubramanian, S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21, 459. [Google Scholar] [CrossRef] [PubMed]
- Abou Assi, H.; Garavıs, M.; Gonzalez, C.; Damha, M.J. i-Motif DNA: Structural features and significance to cell biology. Nucleic Acids Res. 2018, 46, 8038. [Google Scholar] [CrossRef] [Green Version]
- Gehring, K.; Mergny, J.L.; Guéron, M. A tetrameric DNA structure with protonated cytosine-cytosine base pairs. Nature 1993, 363, 561. [Google Scholar] [CrossRef] [PubMed]
- Mergny, J.L.; Sen, D. DNA Quadruple Helices in Nanotechnology. Chem. Rev. 2019, 119, 6290. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.P.; Huppert, J.L.; Waller, Z.A.E. Identification of multiple genomic DNA sequences which form i-motif structures at neutral pH. Nucleic Acids Res. 2017, 45, 2951. [Google Scholar] [CrossRef] [Green Version]
- Skolakova, P.; Foldynova-Trantirkova, S.; Bednarova, K.; Fiala, R.; Vorlickova, M.; Trantirek, L. Unique C. elegans telomeric overhang structures reveal the evolutionarily conserved properties of telomeric DNA. Nucleic Acids Res. 2015, 43, 4733. [Google Scholar] [CrossRef] [Green Version]
- Oganesian, L.; Karlseder, J. Mammalian 5′ C-rich telomeric overhangs are a mark of recombination-dependent telomere maintenance. Mol. Cell 2011, 42, 224. [Google Scholar] [CrossRef] [Green Version]
- Dzatko, S.; Krafcikova, M.; Hänsel-Hertsch, R.; Fessl, T.; Fiala, R.; Loja, T.; Krafcik, D.; Mergny, J.L.; Foldynova-Trantirkova, S.; Trantirek, L. Evaluation of the stability of DNA i-Motifs in the nuclei of living mammalian cells. Angew. Chem. Int. Ed. Engl. 2018, 57, 2165. [Google Scholar] [CrossRef] [Green Version]
- Zeraati, M.; Langley, D.B.; Schofield, P.; Moye, A.L.; Rouet, R.; Hughes, W.E.; Bryan, T.M.; Dinger, M.E.; Christ, D. I-motif DNA structures are formed in the nuclei of human cells. Nat. Chem. 2018, 10, 631. [Google Scholar] [CrossRef]
- Day, H.A.; Pavlos, P.; Waller, Z.A.E. i-Motif DNA: Structure, stability and targeting with ligands. Bioorg. Med. Chem. 2014, 22, 4407. [Google Scholar] [CrossRef]
- Masoud, S.S.; Nagasawa, K. i-Motif-binding ligands and their effects on the structure and biological functions of i-Motif. Chem. Pharm. Bull. 2018, 66, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, J.J.; Irving, K.L.; Evans, C.W.; Chikhale, R.V.; Becker, R.; Morris, C.J.; Pena Martinez, C.D.; Schofield, P.; Christ, D.; Hurley, L.H.; et al. DNA G-Quadruplex and i-Motif Structure Formation Is Interdependent in Human Cells. J. Am. Chem. Soc. 2020, 142, 20600. [Google Scholar] [CrossRef]
- Martino, L.; Pagano, B.; Fotticchia, I.; Neidle, S.; Giancola, C. Shedding light on the interaction between TMPyP4 and human telomeric quadruplexes. J. Phys. Chem. B 2009, 113, 14779–14786. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, O.Y.; Rangan, A.; Chemeris, V.V.; Hurley, L.H. Cationic porphyrins promote the formation of i-motif DNA and bind peripherally by a non-intercalative mechanism. Biochemistry 2000, 39, 15083. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.P.; Day, H.A.; Ibrahim, A.M.; Kumar, J.; Boswell, L.J.E.; Huguin, C.; Stevenson, C.E.M.; Pors, K.; Waller, Z.A.E. Mitoxantrone and analogues bind and stabilize i-motif forming DNA sequences. Sci. Rep. 2016, 6, 39456. [Google Scholar] [CrossRef] [Green Version]
- Pages, B.J.; Gurung, S.P.; McQuaid, K.; Hall, J.P.; Cardin, C.J.; Brazier, J.A. Stabilization of long-looped i-motif DNA by polypyridyl ruthenium complexes. Front. Chem. 2019, 7, 744. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Hong, S.; Sun, N.; Wang, K.; Zhou, L.; Jia, L.; Pei, R. Berberine as a novel light-up i-motif fluorescence ligand and its application in designing molecular logic systems. Chem. Commun. 2016, 52, 179. [Google Scholar] [CrossRef]
- Bonnet, H.; Morel, M.; Devaux, A.; Boissieras, J.; Granzhan, A.; Elias, B.; Lavergne, T.; Dejeu, J.; Defrancq, E. Assessment of presumed small-molecule ligands of telomeric i-DNA by biolayer interferometry (BLI). Chem. Commun. 2022, 58, 5116. [Google Scholar] [CrossRef]
- Gargallo, R.; Avino, A.; Eritja, R.; Jarosova, P.; Mazzini, S.; Scaglioni, L.; Taborsky, P. Study of alkaloid berberine and its interaction with the human telomeric i-motif DNA structure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119185. [Google Scholar] [CrossRef]
- Kendrick, S.; Kang, H.J.; Alam, M.P.; Madathil, M.M.; Agrawal, P.; Gokhale, V.; Yang, D.; Hecht, S.M.; Hurley, L.H. The dynamic character of the BCL2 promoter i-motif provides a mechanism for modulation of gene expression by compounds that bind selectively to the alternative DNA hairpin structure. J. Am. Chem. Soc. 2014, 136, 4161. [Google Scholar] [CrossRef]
- Kang, H.J.; Kendrick, S.; Hecht, S.M.; Hurley, L.H. The transcriptional complex between the BCL2 i-motif and hnRNP LL ss a molecular switch for control of gene expression that can be modulated by small molecules. J. Am. Chem. Soc. 2014, 136, 4172. [Google Scholar] [CrossRef]
- Gillard, M.; Laramée-Milette, B.; Deraedt, Q.; Hanan, G.S.; Loiseau, F.; Dejeu, J.; Defrancq, E.; Elias, B.; Marcélis, L. Photodetection of DNA mismatches by dissymmetric Ru(II) acridine based complexes. Inorg. Chem. Front. 2019, 6, 2260. [Google Scholar] [CrossRef]
- Grimley, E.; Liao, C.; Ranghini, E.J.; Nikolovska-Coleska, Z.; Dressler, G.R. Inhibition of Pax2 Transcription Activation with a Small Molecule that Targets the DNA Binding Domain. ACS Chem. Biol. 2017, 12, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weynand, J.; Bonnet, H.; Loiseau, F.; Ravanat, J.L.; Dejeu, J.; Defrancq, E.; Elias, B. Targeting G-rich DNA structures with photo-reactive bis-cyclometalated iridium(III) complexes. Chem. Eur. J. 2019, 25, 12730. [Google Scholar] [CrossRef]
- Gillard, M.; Weynand, J.; Bonnet, H.; Loiseau, F.; Decottignies, A.; Dejeu, J.; Defrancq, E.; Elias, B. Flexible RuII schiff base complexes: G-quadruplex DNA binding and photo-induced cancer cell death. Chem. Eur. J. 2020, 26, 13849. [Google Scholar] [CrossRef] [PubMed]
- Arnell, R.; Ferraz, N.; Fornstedt, T. Analytical characterization of chiral drug-protein interactions: Comparison between the optical biosensor (surface plasmon resonance) assay and the HPLC perturbation method. Anal. Chem. 2006, 78, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Tsatsas, G.; Vassiliadou, N. Synthèse de quelques dérivés dialcoylaminoacétylés des 3α- et 3β-aminocholestanes. C. R. Acad. Sci. Paris 1964, 259, 1972. [Google Scholar]
- Salehi, P.; Khodaei, M.M.; Zolfigol, M.A.; Keyvan, A. Facile conversion of alcohols into N-substituted amides by magnesium hydrogenosulfate under heterogeneous conditions. Synth. Commun. 2001, 31, 1947. [Google Scholar] [CrossRef]
- Tsvetkov, V.B.; Turaev, A.V.; Petrunina, N.A.; Melnik, D.M.; Khodarovich, Y.M.; Pozmogova, G.E.; Zatsepin, T.S.; Varizhuk, A.M.; Aralov, A.V. Phenoxazine pseudonucleotides in DNA i-motifs allow precise profiling of small molecule binders by fluorescence monitoring. Analyst 2021, 146, 4436. [Google Scholar] [CrossRef]
- Pagano, A.; Iaccarino, N.; Abdelhamid, M.A.S.; Brancaccio, D.; Garzarella, E.U.; Di Porzio, A.; Novellino, E.; Waller, Z.A.E.; Pagano, B.; Amato, J.; et al. Common G-quadruplex binding agents found to interact with i-motif-forming DNA: Unexpected multi-target-directed compounds. Front. Chem. 2018, 6, 281. [Google Scholar] [CrossRef]
- Kypr, J.; Kejnovska, I.; Renciuk, D.; Vorlickova, M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713. [Google Scholar] [CrossRef] [PubMed]
- This sequence is derived from DAP sequence and is unable to fold into an i-motif (see CD spectra in the Supporting Information).
- Molnar, M.M.; Liddell, S.C.; Wadkins, R.M. Effects of polyamine binding on the stability of DNA i-motif structures. ACS Omega 2019, 4, 8967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhavsar-Jog, Y.P.; Van Dornshuld, E.V.; Brooks, T.A.; Tschumper, G.S.; Wadkins, R.M. Epigenetic Modification, Dehydration, and Molecular Crowding Effects on the Thermodynamics of i-Motif Structure Formation from C-Rich DNA. Biochemistry 2014, 53, 1586. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.I.; Young, R.A. Transcriptional regulation and its misregulation in disease. Cell 2013, 52, 1237–1251. [Google Scholar] [CrossRef] [Green Version]
- Shaterian, H.R.; Hosseinian, A.; Ghashang, M. One-pot preparation of β-amido ketones/esters in a three-component condensation reaction using magnesium hydrogensulfate as an effective and reusable catalyst. Can. J. Chem. 2008, 86, 376. [Google Scholar] [CrossRef]






| Acronym | Sequence (5′ → 3′) |
|---|---|
| c-myc | TCCCCACCTTCCCCACCCTCCCCACCCTCCCCA |
| HRAS | CGCCCGTGCCCTGCGCCCGCAACCCGA |
| Bcl-2 | CAGCCCCGCTCCCGCCCCCTTCCTCCCGCGCCCGCCCCT |
| Htelo-C | TAACCCTAACCCTAACCCTAACCCTAA |
| ssDNA | GGCATAGTGCGTGGGCG |
| HP-GC | CGCGCGCGTTTTCGCGCGCG |
| DAP-Mut | CTACCGCTAACGCCTTCGCTCTCGCTTTC |
| Sequence | ∆Tm/°C a | ∆pHT b | ||
|---|---|---|---|---|
| Denaturation | Renaturation | Denaturation | Renaturation | |
| HRAS | −1.2 ± 1.1 | −0.5 ± 1.1 | −0.02 ± 0.15 | −0.04 ± 0.06 |
| bcl-2 | 0.2 ± 0.4 | 0.2 ± 0.9 | 0.01 ± 0.08 | −0.02 ± 0.03 |
| HTelo-C | −1.2 ± 0.5 | −0.7 ± 0.9 | 0.10 ± 0.04 | −0.06 ± 0.08 |
| c-myc | −0.5 ± 0.1 | −1.1 ± 1.2 | 0.03 ± 0.06 | −0.11 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthiol, F.; Boissieras, J.; Bonnet, H.; Pierrot, M.; Philouze, C.; Poisson, J.-F.; Granzhan, A.; Dejeu, J.; Defrancq, E. Novel Synthesis of IMC-48 and Affinity Evaluation with Different i-Motif DNA Sequences. Molecules 2023, 28, 682. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020682
Berthiol F, Boissieras J, Bonnet H, Pierrot M, Philouze C, Poisson J-F, Granzhan A, Dejeu J, Defrancq E. Novel Synthesis of IMC-48 and Affinity Evaluation with Different i-Motif DNA Sequences. Molecules. 2023; 28(2):682. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020682
Chicago/Turabian StyleBerthiol, Florian, Joseph Boissieras, Hugues Bonnet, Marie Pierrot, Christian Philouze, Jean-François Poisson, Anton Granzhan, Jérôme Dejeu, and Eric Defrancq. 2023. "Novel Synthesis of IMC-48 and Affinity Evaluation with Different i-Motif DNA Sequences" Molecules 28, no. 2: 682. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28020682