Achieving Luminescence of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+ via [In3+] Substitution [Ga3+] and Its Application to NIR pc-LED in Non-Destructive Testing
Abstract
:1. Introduction
2. Results and Discussion
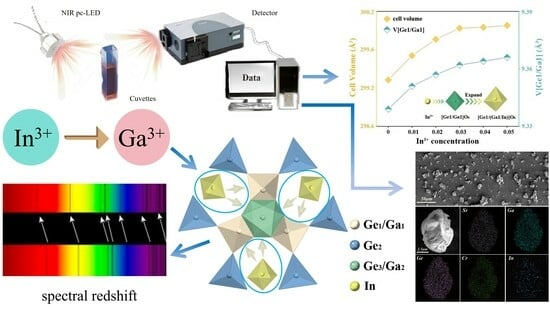
2.1. Phase Purity and Crystal Structure Analysis
2.2. Luminescence Characterization and Mechanism of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+
2.3. Temperature Stability and Quantum Efficiency Analysis of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+
2.4. Application Exploration
3. Materials and Methods
3.1. Sample Preparation
3.2. Preparation of pc-LED
3.3. Sample Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, D.; Zhu, H.; Deng, Z.; Yang, H.; Hu, J.; Liang, S.; Chen, D.; Ma, E.; Guo, W. A highly efficient and thermally stable broadband Cr3+-activated double borate phosphor for near-infrared light-emitting diodes. J. Mater. Chem. C 2021, 9, 164–172. [Google Scholar] [CrossRef]
- Miao, S.; Liang, Y.; Zhang, Y.; Chen, D.; Wang, X.-J. Broadband short-wave infrared light-emitting diodes based on Cr3+-doped LiScGeO4 phosphor. ACS Appl. Mater. Interfaces 2021, 13, 36011–36019. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Wang, S.; Su, J.; Yuan, W.; Wu, H.; Pang, R.; Wang, J.; Li, C.; Zhang, H. Design of a novel near-infrared luminescence material Li2Mg3TiO6:Cr3+ with an ultrawide tuning range applied to near-infrared light-emitting diodes. ACS Sustain. Chem. Eng. 2022, 10, 3839–3850. [Google Scholar] [CrossRef]
- Zhong, J.; Zhuo, Y.; Du, F.; Zhang, H.; Zhao, W.; Brgoch, J. Efficient and Tunable Luminescence in Ga2−xInxO3:Cr3+ for Near-Infrared Imaging. ACS Appl. Mater. Interfaces 2021, 13, 31835–31842. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cai, H.; Song, Z.; Liu, Q. Structural confinement for Cr3+ activators toward efficient near-infrared phosphors with suppressed concentration quenching. Chem. Mater. 2021, 33, 3621–3630. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Zhao, Y.; Gong, Z.; Zhang, M.; Yan, D.; Zhu, H.; Liu, C.; Xu, C.; Zhang, H. Ratiometric afterglow nanothermometer for simultaneous in situ bioimaging and local tissue temperature sensing. Chem. Mater. 2017, 29, 8119–8131. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wu, X.; Huang, L.; Li, D.; Fan, W.; Han, G. Direct aqueous-phase synthesis of sub-10 nm “luminous pearls” with enhanced in vivo renewable near-infrared persistent luminescence. J. Am. Chem. Soc. 2015, 137, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Katayama, Y.; Ueda, J.; Tanabe, S. A brief review on red to near-infrared persistent luminescence in transition-metal-activated phosphors. Opt. Mater. 2014, 36, 1907–1912. [Google Scholar] [CrossRef]
- Chen, H.-W.; Zhu, R.-D.; He, J.; Duan, W.; Hu, W.; Lu, Y.-Q.; Li, M.-C.; Lee, S.-L.; Dong, Y.-J.; Wu, S.-T. Going beyond the limit of an LCD’s color gamut. Light Sci. Appl. 2017, 6, e17043. [Google Scholar] [CrossRef]
- Song, E.; Jiang, X.; Zhou, Y.; Lin, Z.; Ye, S.; Xia, Z.; Zhang, Q. Heavy Mn2+ doped MgAl2O4 phosphor for high-efficient near-infrared light-emitting diode and the night-vision application. Adv. Opt. Mater. 2019, 7, 1901105. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Hao, Z.; Zhang, X.; Pan, G.-h.; Luo, Y.; Wu, H.; Zhang, J. A high efficiency broad-band near-infrared Ca2LuZr2Al3O12:Cr3+ garnet phosphor for blue LED chips. J. Mater. Chem. C 2018, 6, 4967–4976. [Google Scholar] [CrossRef]
- Pan, L.; Lu, R.; Zhu, Q.; McGrath, J.M.; Tu, K. Measurement of moisture, soluble solids, sucrose content and mechanical properties in sugar beet using portable visible and near-infrared spectroscopy. Postharvest Biol. Technol. 2015, 102, 42–50. [Google Scholar] [CrossRef]
- Ye, M.; Gao, Z.; Li, Z.; Yuan, Y.; Yue, T. Rapid detection of volatile compounds in apple wines using FT-NIR spectroscopy. Food Chem. 2016, 190, 701–708. [Google Scholar] [CrossRef]
- Sarkar, S.; Le, P.; Geng, J.; Liu, Y.; Han, Z.; Zahid, M.U.; Nall, D.; Youn, Y.; Selvin, P.R.; Smith, A.M. Short-wave infrared quantum dots with compact sizes as molecular probes for fluorescence microscopy. J. Am. Chem. Soc. 2020, 142, 3449–3462. [Google Scholar] [CrossRef] [PubMed]
- Zabiliūtė, A.; Butkutė, S.; Žukauskas, A.; Vitta, P.; Kareiva, A. Sol-gel synthesized far-red chromium-doped garnet phosphors for phosphor-conversion light-emitting diodes that meet the photomorphogenetic needs of plants. Appl. Opt. 2014, 53, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Guelpa, A.; Marini, F.; du Plessis, A.; Slabbert, R.; Manley, M. Verification of authenticity and fraud detection in South African honey using NIR spectroscopy. Food Control 2017, 73, 1388–1396. [Google Scholar] [CrossRef]
- Elzbieciak-Piecka, K.; Matuszewska, C.; Marciniak, L. Step by step designing of sensitive luminescent nanothermometers based on Cr3+, Nd3+ co-doped La3−xLuxAl5−yGayO12 nanocrystals. New J. Chem. 2019, 43, 12614–12622. [Google Scholar] [CrossRef]
- Bai, B.; Dang, P.; Zhu, Z.; Lian, H.; Lin, J. Broadband near-infrared emission of La3Ga5GeO14:Tb3+, Cr3+ phosphors: Energy transfer, persistent luminescence and application in NIR light-emitting diodes. J. Mater. Chem. C 2020, 8, 11760–11770. [Google Scholar] [CrossRef]
- Cui, J.; Li, P.; Cao, L.; Wang, X.; Yao, Y.; Zhang, M.; Zheng, M.; Yang, Z.; Suo, H.; Wang, Z. Achievement of broadband near-infrared phosphor Ca3Y2Ge3O12:Cr3+, Ce3+ via energy transfer for food analysis. J. Lumin. 2021, 237, 118170. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Y.; Ren, J.; Fang, Z.; Lu, X.; Lewis, E.; Farrell, G.; Yang, J.; Wang, P. Selective doping of Ni2+ in highly transparent glass-ceramics containing nano-spinels ZnGa2O4 and Zn1+xGa2−2xGexO4 for broadband near-infrared fiber amplifiers. Sci. Rep. 2017, 7, 1783. [Google Scholar] [CrossRef]
- Cao, L.; Li, P.; Cui, J.; Wang, X.; Yao, Y.; Zhang, M.; Zheng, M.; Yang, Z.; Suo, H.; Wang, Z. Achieving the potential multifunctional near-infrared materials Ca3In2−xGaxGe3O12:Cr3+ using a solid state method. RSC Adv. 2021, 11, 10043–10053. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Molokeev, M.S.; Lei, B.; Xia, Z. Two-site Cr3+ occupation in the MgTa2O6:Cr3+ phosphor toward broad-band near-infrared emission for vessel visualization. J. Mater. Chem. C 2020, 8, 9322–9328. [Google Scholar] [CrossRef]
- Petermüller, B.; Petschnig, L.L.; Wurst, K.; Heymann, G.; Huppertz, H. Synthesis and Characterization of the New Strontium Borogermanate Sr3−x/2B2−xGe4+xO14 (x = 0.32). Inorg. Chem. 2014, 53, 9722–9728. [Google Scholar] [CrossRef] [PubMed]
- Basore, E.T.; Xiao, W.; Liu, X.; Wu, J.; Qiu, J. Broadband near-infrared garnet phosphors with near-unity internal quantum efficiency. Adv. Opt. Mater. 2020, 8, 2000296. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Wang, Y.; Shi, Y.; He, S.; Yang, Y.; Li, R.; Wei, G.; Suo, H.; Wang, Z. Two-stage ultra-broadband luminescence of Cr3+-doped multisite layered phosphor Sr3Ga2Ge4O14 and its application in pc-LEDs. Mater. Today Chem. 2022, 26, 101102. [Google Scholar] [CrossRef]
- Adachi, S. photoluminescence properties of Cr3+-activated oxide phosphors. ECS J. Solid State Sci. Technol. 2021, 10, 026001. [Google Scholar] [CrossRef]
- Chi, F.; Dai, W.; Liu, S.; Qiu, L.; Wei, X.; Chen, Y.; Yin, M. Luminescence properties of Cr3+-doped Al6Ge2O13 broadband near-infrared phosphor. Opt. Mater. 2022, 126, 112218. [Google Scholar] [CrossRef]
- Ma, C.; Wang, M.; Xue, B.; Lu, B. Structural features and photoluminescence behaviors of color-temperature tunable (Gd,Y)3Al5O12:Ce3+/Cr3+ phosphors with varying compositions. J. Non-Cryst. Solids 2022, 588, 121645. [Google Scholar] [CrossRef]
- Mao, N.; Liu, S.; Song, Z.; Yu, Y.; Liu, Q. A broadband near-infrared phosphor Ca3Y2Ge3O12:Cr3+ with garnet structure. J. Alloys Compd. 2021, 863, 158699. [Google Scholar] [CrossRef]
- Duong, L.; Tuan, N.; Quang, N.; Huy, P.T.; Nguyen, D.H. Synthesis and photoluminescence properties of deep-red-emitting cayalo4:Cr3+ phosphors. J. Electron. Mater. 2020, 49, 7464–7471. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, J.; Du, F.; Chen, L.; Zhang, X.; Mu, Z.; Zhao, W. Efficient and thermally stable broad-band near-infrared emission in a KAlP2O7:Cr3+ phosphor for nondestructive examination. ACS Appl. Mater. Interfaces 2022, 14, 11663–11671. [Google Scholar] [CrossRef]
- Zhao, F.; Cai, H.; Zhang, S.; Song, Z.; Liu, Q. Octahedron-dependent near-infrared luminescence in Cr3+-activated phosphors. Mater. Today Chem. 2022, 23, 100704. [Google Scholar] [CrossRef]
- Adachi, S. Properties of Semiconductor Alloys: Group-IV, III-V and II-VI Semiconductors; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Liu, H.; Zhao, F.; Cai, H.; Song, Z.; Liu, Q. Consequence of optimal bonding on cation ordering and enhanced near-infrared luminescence in Cr3+-doped pyroxene oxides. J. Mater. Chem. C 2022, 10, 9232–9240. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Wang, Y. Crystal-field engineering control of an ultraviolet–visible-responsive near-infrared-emitting phosphor and its applications in plant growth, night vision, and NIR spectroscopy detection. Adv. Opt. Mater. 2022, 10, 2102246. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Zhang, L.; Wu, H.; Zhang, J. Cr3+ Activated Garnet Phosphor with Efficient Blue to Far-Red Conversion for pc-LED. Adv. Opt. Mater. 2021, 9, 2101134. [Google Scholar] [CrossRef]
- Wu, X.; Xu, D.; Li, W.; Wang, T.; Cao, L.; Meng, J. Synthesis and luminescence of novel near-infrared emitting BaZrSi3O9:Cr3+ phosphors. Luminescence 2017, 32, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xia, Z.; Molokeev, M.S.; Liu, Q.; Guo, H. Synthesis, Crystal Structure, and Enhanced Luminescence of Garnet-Type Ca3Ga2Ge3O12:Cr3+ by Codoping Bi3+. J. Am. Ceram. Soc. 2015, 98, 1870–1876. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sugano, S. On the absorption spectra of complex ions II. J. Phys. Soc. Jpn. 1954, 9, 766–779. [Google Scholar] [CrossRef]
- Adachi, S. New analysis model for the determination of racah and crystal-field splitting parameters: Verification and case studies. ECS J. Solid State Sci. Technol. 2020, 9, 046004. [Google Scholar] [CrossRef]
- Leśniewski, T. Evolution of the full energy structure of Mn4+ in fluoride phosphors under high pressure conditions. Phys. Chem. Chem. Phys. 2023, 25, 14449–14462. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S. Mn4+ and Cr3+ ions in red and deep red-emitting phosphors: Spectral analysis and Racah parameter determination. J. Lumin. 2020, 223, 117217. [Google Scholar] [CrossRef]
- Adachi, S. Temperature dependence of luminescence intensity and decay time in Cr3+-activated oxide and fluoride phosphors. ECS J. Solid State Sci. Technol. 2022, 11, 066001. [Google Scholar] [CrossRef]
- Wen, D.; Liu, H.; Guo, Y.; Zeng, Q.; Wu, M.; Liu, R.S. Disorder–Order Conversion-Induced Enhancement of Thermal Stability of Pyroxene Near-Infrared Phosphors for Light-Emitting Diodes. Angew. Chem. Int. Ed. 2022, 61, e202204411. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Hao, Z.; Zhang, X.; Pan, G.h.; Wu, H.; Zhang, J. Cr3+-doped broadband NIR garnet phosphor with enhanced luminescence and its application in NIR spectroscopy. Adv. Opt. Mater. 2019, 7, 1900185. [Google Scholar] [CrossRef]
- Xiang, J.; Zheng, J.; Zhao, X.; Zhou, X.; Chen, C.; Jin, M.; Guo, C. Synthesis of broadband NIR garnet phosphor Ca4ZrGe3O12:Cr3+, Yb3+ for NIR pc-LED applications. Mater. Chem. Front. 2022, 6, 440–449. [Google Scholar] [CrossRef]
- Lang, T.; Cai, M.; Fang, S.; Han, T.; He, S.; Wang, Q.; Ge, G.; Wang, J.; Guo, C.; Peng, L. Trade-off Lattice Site Occupancy Engineering Strategy for Near-Infrared Phosphors with Ultrabroad and Tunable Emission. Adv. Opt. Mater. 2022, 10, 2101633. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Gao, B.; Li, J.; Wang, Z.; Li, P. Achieving Luminescence of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+ via [In3+] Substitution [Ga3+] and Its Application to NIR pc-LED in Non-Destructive Testing. Molecules 2023, 28, 8059. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28248059
Wang T, Gao B, Li J, Wang Z, Li P. Achieving Luminescence of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+ via [In3+] Substitution [Ga3+] and Its Application to NIR pc-LED in Non-Destructive Testing. Molecules. 2023; 28(24):8059. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28248059
Chicago/Turabian StyleWang, Tao, Bingkai Gao, Jiehong Li, Zhijun Wang, and Panlai Li. 2023. "Achieving Luminescence of Sr3Ga1.98In0.02Ge4O14:0.03Cr3+ via [In3+] Substitution [Ga3+] and Its Application to NIR pc-LED in Non-Destructive Testing" Molecules 28, no. 24: 8059. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28248059