Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Properties
2.2. FT-IR Spectroscopy
2.3. Light Microscopy
2.4. Water Absorption Properties
2.5. Application on Soil
2.5.1. Morphological Analysis of the Soil
2.5.2. Evaluation of the Maximum Water Holding Capacity (MWHC) of the Soil
2.5.3. Evaluation of the Water Retention Capacity of the Soil
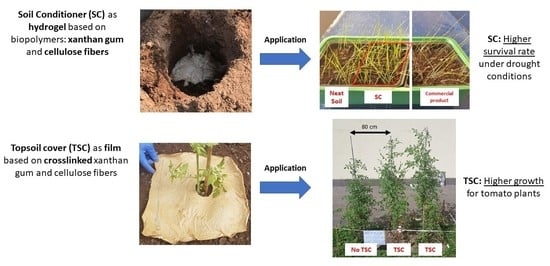
2.5.4. Case Study Application
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Characterization
3.3.1. Rheological Properties
3.3.2. FT-IR Spectroscopy
3.3.3. Light Microscopy
3.3.4. Water Absorption Properties
3.3.5. Application on the Soil
Morphological Analysis of the Soil
Evaluation of the Maximum Water Holding Capacity (MWHC) of the Soil
Evaluation of the Water Retention Capacity of the Soil
Case Study Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Schelhaas, M.J.; Nabuurs, G.J.; Schuck, A. Natural disturbances in the European forests in the 19th and 20th centuries. Global Chang. Biol. 2003, 9, 1620–1633. [Google Scholar] [CrossRef]
- Fischer, A.; Lindner, M.; Abs, C.; Lasch, P. Vegetation dynamics in central European forest ecosystems (near-natural as well as managed) after storm events. Folia Geobot. 2002, 37, 17–32. [Google Scholar] [CrossRef]
- Hlásny, T.; König, L.; Krokene, P.; Lindner, M.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.-J.; Svoboda, M. Bark beetle outbreaks in Europe: State of knowledge and ways forward for management. Curr. For. Rep. 2021, 7, 138–165. [Google Scholar] [CrossRef]
- Jakoby, O.; Lischke, H.; Wermelinger, B. Climate change alters elevational phenology patterns of the European spruce bark beetle (Ips typographus). Glob. Chang. Biol. 2019, 25, 4048–4063. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, V.R.n.; Smanis, A.; Chirino, E.; Fuentes, D.; Valdecantos, A.; Vilagrosa, A. Perspectives in dryland restoration: Approaches for climate change adaptation. New For. 2012, 43, 561–579. [Google Scholar] [CrossRef] [Green Version]
- Bertini, G.; Amoriello, T.; Fabbio, G.; Piovosi, M. Forest growth and climate change: Evidences from the ICP-Forests intensive monitoring in Italy. Iforest 2011, 4, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo-Comino, J.; Salvia, R.; Egidi, G.; Salvati, L.; Giménez-Morera, A.; Quaranta, G. Desertification and Degradation Risks vs. Poverty: A Key Topic in Mediterranean Europe. Cuad. Investig. Geogr. 2022, 48, 23–40. [Google Scholar] [CrossRef]
- Shakesby, R. Post-wildfire soil erosion in the Mediterranean: Review and future research directions. Earth-Sci. Rev. 2011, 105, 71–100. [Google Scholar] [CrossRef]
- Dennis, E.S.; Dolferus, R.; Ellis, M.; Rahman, M.; Wu, Y.; Hoeren, F.U.; Grover, A.; Ismond, K.P.; Good, A.G.; Peacock, W.J. Molecular strategies for improving waterlogging tolerance in plants. J. Exp. Bot. 2000, 51, 89–97. [Google Scholar] [CrossRef]
- Farooq, M.; Ihsan, J.; Saeed, S.; Haleem, A.; Siddiq, M. Highly Versatile Gum Acacia Based Swellable Microgels Encapsulating Cobalt Nanoparticles; An Approach to Rapid and Recoverable Environmental Nano-catalysis. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2030–2042. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef] [Green Version]
- Ihsan, J.; Farooq, M.; Khan, M.A.; Khan, A.S.; Muhammad, S.; Ahmad, N.; Haleem, A.; Shah, L.A.; Saeed, S.; Siddiq, M. Acacia Gum Hydrogels Embedding the In Situ Prepared Silver Nanoparticles; Synthesis, Characterization, and Catalytic Application. Catal. Lett. 2020, 151, 1212–1223. [Google Scholar] [CrossRef]
- Coello, J.; Piqué, M. Soil Conditioners and Groundcovers for Sustainable and Cost-Efficient Tree Planting in Europe and the Mediterranean-Technical Guide; Centre Tecnològic Forestal de Catalunya: Solsona, Spain, 2016; p. 60. [Google Scholar]
- Coello, J.; Ameztegui, A.; Rovira, P.; Fuentes, C.; Piqué, M. Innovative soil conditioners and mulches for forest restoration in semiarid conditions in northeast Spain. Ecol. Eng. 2018, 118, 52–65. [Google Scholar] [CrossRef]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sojka, R.; Bjorneberg, D.; Entry, J.; Lentz, R.; Orts, W. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Ritonga, H.; Nurfadillah, A.; Rembon, F.S.; Ramadhan, L.O.A.N.; Nurdin, M. Preparation of Chitosan-EDTA hydrogel as soil conditioner for soybean plant (Glycine max). Groundw. Sustain. Dev. 2019, 9, 100277. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Water sorption kinetics of superabsorbent hydrogels of saponified cassava starch-graft-poly(acrylamide). Starke 2012, 64, 803–812. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hosseinzadeh, H. Synthesis and superswelling behavior of carboxymethylcellulose–poly(sodium acrylate-co-acrylamide) hydrogel. J. Appl. Polym. Sci. 2008, 108, 1142–1151. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Samadi, M.; Ghasemzadeh, H. Fast-swelling Superabsorbent Hydrogels from Poly(2-hydroxy ethyl acrylate-co-sodium acrylate) Grafted on Starch. Starke 2008, 60, 79–86. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Fajardo, A.R.; Muniz, E.C.; Tambourgi, E.B. Superabsorbent hydrogel based on modified polysaccharide for removal of Pb2+ and Cu2+ from water with excellent performance. J. Appl. Polym. Sci. 2007, 105, 2903–2909. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Zhang, L.; Zhan, H. Superabsorbent nanocomposite hydrogels made of carboxylated cellulose nanofibrils and CMC-g-p(AA-co-AM). Carbohydr. Polym. 2013, 97, 429–435. [Google Scholar] [CrossRef]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Theodoropoulos, A.G.; Drossopoulos, J.B. Designing synthetic polymers as soil conditioners. Commun. Soil Sci. Plant Anal. 2008, 26, 1455–1480. [Google Scholar] [CrossRef]
- Orts, W.J.; Sojka, R.E.; Glenn, G.M.J.I.C. Biopolymer additives to reduce erosion-induced soil losses during irrigation. Ind. Crops Prod. 2000, 11, 19–29. [Google Scholar] [CrossRef]
- Chang, I.; Prasidhi, A.K.; Im, J.; Shin, H.-D.; Cho, G.-C. Soil treatment using microbial biopolymers for anti-desertification purposes. Geoderma 2015, 253–254, 39–47. [Google Scholar] [CrossRef]
- Machado, W.; Figueiredo, A.; Guimarães, M.F. Initial development of seedlings of macauba palm (Acrocomia aculeata). Ind. Crops Prod. 2016, 87, 14–19. [Google Scholar] [CrossRef]
- Benigno, S.M.; Dixon, K.W.; Stevens, J.C. Increasing Soil Water Retention with Native-Sourced Mulch Improves Seedling Establishment in Postmine Mediterranean Sandy Soils. Restor. Ecol. 2013, 21, 617–626. [Google Scholar] [CrossRef]
- Dearfield, K.L.; Abernathy, C.O.; Ottley, M.S.; Brantner, J.H.; Hayes, P.F. Acrylamide: Its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutat. Res.-Rev. Genet. Toxicol. 1988, 195, 45–77. [Google Scholar] [CrossRef]
- Christensen, L.H.; Breiting, V.B.; Aasted, A.; Jørgensen, A.; Kebuladze, I. Long-Term Effects of Polyacrylamide Hydrogel on Human Breast Tissue. Plast. Reconstr. Surg. 2003, 111, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Zou, H.-C.; Lü, Z.-R.; Zou, F.; Park, Y.-D.; Yan, Y.-B.; Yao, S.-J. Effects of Acrylamide on the Activity and Structure of Human Brain Creatine Kinase. Int. J. Mol. Sci. 2009, 10, 4210–4222. [Google Scholar] [CrossRef] [PubMed]
- Cabalar, A.F.; Awraheem, M.H.; Khalaf, M.M. Geotechnical Properties of a Low-Plasticity Clay with Biopolymer. J. Mater. Civ. Eng. 2018, 30, 04018170. [Google Scholar] [CrossRef]
- Lentz, R.D. Polyacrylamide and biopolymer effects on flocculation, aggregate stability, and water seepage in a silt loam. Geoderma 2015, 241–242, 289–294. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.-M.; Im, J.; Cho, G.-C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuang, J.; Wang, Y.; Jia, Y.; Niu, P.; Jia, K. Improvement of loess characteristics using sodium alginate. Bull. Eng. Geol. Environ. 2019, 79, 1879–1891. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Langford, S.J.; Ahmad, M.B.; Lim, Y.Y.; Hashim, K. Eco-friendly smart hydrogels for soil conditioning and sustain release fertilizer. Int. J. Environ. Sci. Technol. 2017, 15, 2059–2074. [Google Scholar] [CrossRef]
- Heise, K.; Kirsten, M.; Schneider, Y.; Jaros, D.; Keller, H.; Rohm, H.; Kalbitz, K.; Fischer, S. From Agricultural Byproducts to Value-Added Materials: Wheat Straw-Based Hydrogels as Soil Conditioners? ACS Sustain. Chem. Eng. 2019, 7, 8604–8612. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Chung, M.-K.; Cho, G.-C. Bovine casein as a new soil strengthening binder from diary wastes. Constr. Build. Mater. 2018, 160, 1–9. [Google Scholar] [CrossRef]
- Dorigato, A.; Negri, M.; Pegoretti, A. Ultrathin wood laminae-polyvinyl alcohol biodegradable composites. Polym. Compos. 2018, 39, 1116–1124. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Dussin, A.; Xanthopoulou, E.; Bikiaris, D.N.; Botta, L.; Fiore, V.; Pegoretti, A. Compatibilization of Polylactide/Poly(ethylene 2,5-furanoate) (PLA/PEF) Blends for Sustainable and Bioderived Packaging. Molecules 2022, 27, 6371. [Google Scholar] [CrossRef] [PubMed]
- Fambri, L.; Dorigato, A.; Pegoretti, A. Role of Surface-Treated Silica Nanoparticles on the Thermo-Mechanical Behavior of Poly(Lactide). Appl. Sci. 2020, 10, 6731. [Google Scholar] [CrossRef]
- Dorigato, A.; Negri, M.; Pegoretti, A. Ultrathin Wood Laminae–Thermoplastic Starch Biodegradable Composites. J. Renew. Mater. 2018, 6, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Valentini, F.; Dorigato, A.; Rigotti, D.; Pegoretti, A. Polyhydroxyalkanoates/Fibrillated Nanocellulose Composites for Additive Manufacturing. J. Polym. Environ. 2019, 27, 1333–1341. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.u.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2021, 9, 104702. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.-C. Effects of Xanthan gum biopolymer on soil strengthening. Constr. Build. Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Moghal, A.A.B.; Vydehi, K.V. State-of-the-art review on efficacy of xanthan gum and guar gum inclusion on the engineering behavior of soils. Innov. Infrastruct. Solut. 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; Gonzalez Lopez, O. Water-soluble polymers in agriculture: Xanthan gum as eco-friendly alternative to synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Gils, P.S.; Ray, D.; Sahoo, P.K. Characteristics of xanthan gum-based biodegradable superporous hydrogel. Int. J. Biol. Macromol. 2009, 45, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Katzen, F.; Pühler, A.; Ielpi, L.J.A.M. biotechnology. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl. Microbiol. Biotechnol. 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Garcıa-Ochoa, F.; Santos, V.; Casas, J.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Maji, B.; Moorthy, N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Ragauskas, A.J. Preparation of superabsorbent cellulosic hydrogels. Carbohydr. Polym. 2012, 87, 1410–1418. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X.L. Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos. Sci. Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Sreekala, M.S.; Goda, K.; Devi, P.V. Sorption characteristics of water, oil and diesel in cellulose nanofiber reinforced corn starch resin/ramie fabric composites. Compos. Interfaces 2008, 15, 281–299. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Bruni, G.P.; Lima, K.O.; Halal, S.; Rosa, G.S.D.; Dias, A.R.G.; Zavareze, E.D.R. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chem. 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Talukdar, M.M.; Vinckier, I.; Moldenaers, P.; Kinget, R. Rheological characterization of xanthan gum and hydroxypropylmethyl cellulose with respect to controlled-release drug delivery. J. Pharm. Sci. 1996, 85, 537–540. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, R.; Xu, W.; Bai, Z.; Zhou, Y.; Zhao, S.; Xu, Y.; Yu, D. Rheological behavior and microstructure of release-controlled hydrogels based on xanthan gum crosslinked with sodium trimetaphosphate. Food Hydrocoll. 2016, 52, 923–933. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Ash, J.; Jia, X.; Leone, A.; Templeton, A. Mechanism and Impact of Excipient Incompatibility: Cross-Linking of Xanthan Gum in Pediatric Powder-for-Suspension Formulations. J. Pharm. Sci. 2019, 108, 3609–3615. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Rahbar, N.; Calvert, P.D. Strong fiber-reinforced hydrogel. Acta Biomater. 2013, 9, 5313–5318. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Sanhueza, C.; Mondaca, P.; Tamayo, M.; Álvaro, J.E.; Díaz-Barrera, A.; Cuneo, I.F. Enhancing the mechanical and hydraulic properties of coarse quartz sand using a water-soluble hydrogel based on bacterial alginate for novel application in agricultural contexts. Soil Sci. Soc. Am. J. 2021, 85, 1880–1893. [Google Scholar] [CrossRef]
- Rettenmaier, J.; Söhne, G. ARBOCEL_R datasheet. 2022. [Google Scholar]
- Bueno, V.B.; Bentini, R.; Catalani, L.H.; Petri, D.F. Synthesis and swelling behavior of xanthan-based hydrogels. Carbohydr. Polym. 2013, 92, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macosko, C.W. Rheology Principles; VCH Publishers, Inc.: New York, NY, USA, 1994; pp. 1–174. [Google Scholar]
- Ni, B.; Liu, M.; Lü, S. Multifunctional slow-release urea fertilizer from ethylcellulose and superabsorbent coated formulations. Chem. Eng. J. 2009, 155, 892–898. [Google Scholar] [CrossRef]



















| Determination | Value |
|---|---|
| Sand (2.0–0.05 mm) | 595 g/kg |
| Silt (0.05–0.002 mm) | 345 g/kg |
| Clay (<0.002 mm) | 60 g/kg |
| pH (in water ratio 1:2.5) | 7.2 |
| Total Limestone | 668 g/kg CaCO3 |
| Active Limestone | 11 g/kg CaCO3 |
| Organic Substance | 257 g/kg |
| Assimilable Phosphorus | 45 mg/kg P2O5 |
| Potassium | 112 mg/kg K2O |
| Magnesium | 2238 mg/kg MgO |
| Sample | Xanthan Gum [wt%] | Cellulose Fibers [wt%] |
|---|---|---|
| X1 | 1.0 | 0.0 |
| X2 | 2.0 | 0.0 |
| X4 | 4.0 | 0.0 |
| X1W2 | 1.0 | 2.0 |
| X2W2 | 2.0 | 2.0 |
| X4W2 | 4.0 | 2.0 |
| X1W5 | 1.0 | 5.0 |
| X2W5 | 2.0 | 5.0 |
| X4W5 | 4.0 | 5.0 |
| Sample | Xanthan Gum [wt%] | Cellulose Fibers [wt%] | Citric Acid [wt%] |
|---|---|---|---|
| TSC_A | 4.0 | 2.0 | 1.0 |
| TSC_B | 4.0 | 2.0 | 2.0 |
| TSC_C | 4.0 | 0.0 | 1.0 |
| TSC_D | 4.0 | 2.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorze, A.; Valentini, F.; Dorigato, A.; Pegoretti, A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules 2023, 28, 1952. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28041952
Sorze A, Valentini F, Dorigato A, Pegoretti A. Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications. Molecules. 2023; 28(4):1952. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28041952
Chicago/Turabian StyleSorze, Alessandro, Francesco Valentini, Andrea Dorigato, and Alessandro Pegoretti. 2023. "Development of a Xanthan Gum Based Superabsorbent and Water Retaining Composites for Agricultural and Forestry Applications" Molecules 28, no. 4: 1952. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules28041952